








生物技术通报 ›› 2021, Vol. 37 ›› Issue (5): 67-75.doi: 10.13560/j.cnki.biotech.bull.1985.2020-0963
谢绍怡( ), 蒋璐蔓, 杨晓峰, 张仙玉, 吴珍芳, 李紫聪(
), 蒋璐蔓, 杨晓峰, 张仙玉, 吴珍芳, 李紫聪( )
)
收稿日期:2020-08-03
出版日期:2021-05-26
发布日期:2021-06-11
作者简介:谢绍怡,女,硕士研究生,研究方向:动物遗传育种与繁殖;E-mail: 基金资助:
XIE Shao-yi( ), JIANG Lu-man, YANG Xiao-feng, ZHANG Xian-yu, WU Zhen-fang, LI Zi-cong(
), JIANG Lu-man, YANG Xiao-feng, ZHANG Xian-yu, WU Zhen-fang, LI Zi-cong( )
)
Received:2020-08-03
Published:2021-05-26
Online:2021-06-11
摘要:
性别控制技术在畜牧业生产、濒危动物保护、伴性疾病预防等方面都有着重要的意义,但是目前较为有效的精子分离技术却因成本高昂、技术难度大的问题无法广泛应用于畜禽生产上。受在蚊子上成功实现性别控制的研究启发,本实验设计了两个靶向小鼠X染色体重复序列的sgRNA,构建了含有靶向切割小鼠X染色体的CRISPR/Cas9系统的CMV载体,并对其进行体外、细胞及胚胎水平上的切割效率验证。结果显示:sgRNA X1和sgRNA X3体外介导的Cas9蛋白切割靶序列的效率分别为60.0%和75.0%;CMV载体成功转染MLTC-1细胞后,死亡率与对照组(转染空载体)相比显著提高;CMV载体对X1靶位点的突变效率为11.1%;与对照组相比,小鼠孤雌胚胎注射CMV载体后囊胚率有一定的下降,但未达到显著水平,而囊胚细胞数则显著下降(P<0.01)。实验证明了CMV载体在体外、细胞及胚胎水平上均具有较高的切割效率,能够成功删除X染色体,可用于减少某一性别的精子或胚胎,从而实现哺乳动物的性别控制。
谢绍怡, 蒋璐蔓, 杨晓峰, 张仙玉, 吴珍芳, 李紫聪. 切割小鼠X染色体的CRISPR/Cas9系统表达载体的构建及验证[J]. 生物技术通报, 2021, 37(5): 67-75.
XIE Shao-yi, JIANG Lu-man, YANG Xiao-feng, ZHANG Xian-yu, WU Zhen-fang, LI Zi-cong. Construction and Verification of CRISPR/Cas9 System Expression Vector for Mouse X Chromosome Cutting[J]. Biotechnology Bulletin, 2021, 37(5): 67-75.
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 产物长度 Product size/bp |
|---|---|---|
| X1-F | TGAGTACTTGGTTAGGGTGAGC | 1 212 |
| X1-R | TGGAAGTCTCTGGCATA | |
| X3-F | AAGGTTCCCAATGGTCACAGG | 1 383 |
| X3-R | ACCATACCATGGTTTTCCCCA |
表1 靶片段扩增引物
Table 1 Target fragment amplification primers
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 产物长度 Product size/bp |
|---|---|---|
| X1-F | TGAGTACTTGGTTAGGGTGAGC | 1 212 |
| X1-R | TGGAAGTCTCTGGCATA | |
| X3-F | AAGGTTCCCAATGGTCACAGG | 1 383 |
| X3-R | ACCATACCATGGTTTTCCCCA |
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 产物长度 Product size/bp |
|---|---|---|
| sgRNA X1-F | TAATACGACTCACTATAGGGG- CTTGGTTAGGGTGAGTGCT | 120 |
| sgRNA X1-R | AAAAGCACCGACTCGGTGCC | |
| sgRNA X3-F | TAATACGACTCACTATAGGG- TAAGTGCTGTGTGCTGCTAC | 120 |
| sgRNA X3-R | AAAAGCACCGACTCGGTGCC |
表2 sgRNA体外转录模板扩增引物
Table 2 Primers for amplification of sgRNA transcription template in vitro
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 产物长度 Product size/bp |
|---|---|---|
| sgRNA X1-F | TAATACGACTCACTATAGGGG- CTTGGTTAGGGTGAGTGCT | 120 |
| sgRNA X1-R | AAAAGCACCGACTCGGTGCC | |
| sgRNA X3-F | TAATACGACTCACTATAGGG- TAAGTGCTGTGTGCTGCTAC | 120 |
| sgRNA X3-R | AAAAGCACCGACTCGGTGCC |
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 产物片段 Product size/bp |
|---|---|---|
| X1 mutation-F | GCATGCTTGGTTAGGGTGAGTT | 954 |
| X1 mutation-R | GCTGGAAACCCAAGCAC |
表3 X1靶位点扩增引物
Table 3 Primers for amplification of the X1 target site
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 产物片段 Product size/bp |
|---|---|---|
| X1 mutation-F | GCATGCTTGGTTAGGGTGAGTT | 954 |
| X1 mutation-R | GCTGGAAACCCAAGCAC |
| sgRNA名称 sgRNA name | 靶位点(拷贝数) Target location(copy number) | 核苷酸序列 Nucleotide sequence(5'-3') |
|---|---|---|
| sgRNA X1 | X1(72) | GCTTGGTTAGGGTGAGTGCT |
| sgRNA X3 | X3(99) | TAAGTGCTGTGTGCTGCTAC |
表4 靶向小鼠X染色体多拷贝基因的sgRNA序列
Table 4 Sequences of sgRNA targeting mouse X chromosome multi-copy genes
| sgRNA名称 sgRNA name | 靶位点(拷贝数) Target location(copy number) | 核苷酸序列 Nucleotide sequence(5'-3') |
|---|---|---|
| sgRNA X1 | X1(72) | GCTTGGTTAGGGTGAGTGCT |
| sgRNA X3 | X3(99) | TAAGTGCTGTGTGCTGCTAC |
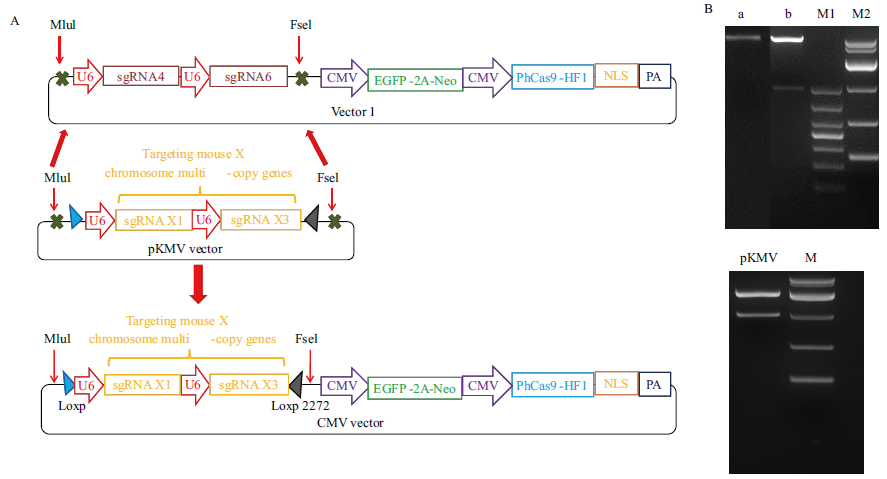
图1 CMV载体的构建及鉴定 A:CMV载体构建示意图。B:载体一和sgRNA片段合成质粒双酶切结果电泳图,M1为1000 DNA marker,M2为10000 DNA Marker;a为原载体一,b为酶切后的载体一;M为10000 DNA marker;pKMV为sgRNA片段合成质粒
Fig.1 Construction and identification of CMV vector A: Schematic diagram of CMV carrier construction. B: Electrophoresis diagram of plasmid double digestion results synthesized by vector 1 and sgRNA fragments, M1 is 1000 DNA marker, M2 is 10000 DNA marker, A is the original vector 1, B is the digested vector 1, M is 10000 DNA marker, and pKMV is a plasmid synthesizing sgRNA fragment
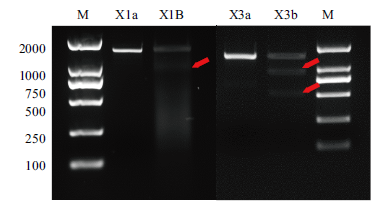
图2 sgRNA X1和sgRNA X3介导Cas9蛋白在体外切割靶序列效率验证的电泳图 图中a为未切割的sgRNA靶序列片段,b为切割后的sgRNA靶序列片段,箭头指示为切割后片段,M为2000 DNA marker
Fig. 2 Electrophoresis diagram of verifying the efficiency of sgRNA X1 and sgRNA X3 mediated Cas9 protein cleaving target sequence in vitro a is the uncut sgRNA target sequence fragment, b is the cut sgRNA target sequence fragment, arrow indicates the cut fragment, and M is 2000 DNA marker

图3 CMV载体、空载体转染MLTC-1细胞不同时间的荧光细胞数比较结果 A:雄性细胞转染CMV载体和空载体后不同时间点的荧光效果图;B:转染载体后不同时间点绿色荧光细胞的比例
Fig.3 Comparison results of the number of fluorescent cells in MLTC-1 cells at different time after transfecting with CMV vector and empty vector A: Fluorescence renderings at different time points after male cells were transfected with CMV vector and empty vector. B: Proportion of green fluorescent cells at different time points after vector transfection

图4 CMV载体转染后对MLTC-1细胞的单克隆细胞团X1靶位点处测序结果 红色框标注的为靶位点,*为匹配碱基,缺失空白处为突变碱基
Fig. 4 Sequencing results of the X1 target site of the monoclonal cell mass of MLTC-1 cells after CMV vector transfection Target sites are marked in the red box, * is the matching base, and the missing space is the mutation base
| 转染细胞 Transfection cell | 检测筛选获得的单克隆细胞团数 Number of monoclonal cell clusters obtained by screening was detected | 突变的单克隆细胞团数 Number of mutant monoclonal cell clusters | 突变效率 Mutation efficiency/% |
|---|---|---|---|
| MLTC-1 | 36 | 4 | 11.1 |
表5 CMV载体转染后MLTC-1细胞X1靶位点的突变效率
Table 5 Mutation efficiency of CMV vector transfected MLTC-1 cell X1 target site
| 转染细胞 Transfection cell | 检测筛选获得的单克隆细胞团数 Number of monoclonal cell clusters obtained by screening was detected | 突变的单克隆细胞团数 Number of mutant monoclonal cell clusters | 突变效率 Mutation efficiency/% |
|---|---|---|---|
| MLTC-1 | 36 | 4 | 11.1 |
| 组别 Group | 注射胚胎数Number of injected embryos | 卵裂胚数Number of cleavage embryo | 卵裂率 Cleavage rate | 囊胚数Number of blastocysts | 囊胚率 Blastocyst rate | 囊胚细胞数 Number of blastocyst cells |
|---|---|---|---|---|---|---|
| 注射 CMV 载体组 The Group of CMV vector injection | 98 | 96 | 98.0% | 57 | 58.1% a | 33.93±4.93 a |
| 注射空载体组 The Group of empty vector injection | 92 | 88 | 95.7% | 64 | 69.5% a | 44.66±6.18 b |
表6 CMV载体和空载体注射孤雌胚体外发育效率比较
Table 6 Comparison of in vitro development efficiency of parthenogenetic embryos injected with CMV vector andempty vector
| 组别 Group | 注射胚胎数Number of injected embryos | 卵裂胚数Number of cleavage embryo | 卵裂率 Cleavage rate | 囊胚数Number of blastocysts | 囊胚率 Blastocyst rate | 囊胚细胞数 Number of blastocyst cells |
|---|---|---|---|---|---|---|
| 注射 CMV 载体组 The Group of CMV vector injection | 98 | 96 | 98.0% | 57 | 58.1% a | 33.93±4.93 a |
| 注射空载体组 The Group of empty vector injection | 92 | 88 | 95.7% | 64 | 69.5% a | 44.66±6.18 b |
| [1] | 李永福. 家畜性别控制技术研究进展[J]. 中国草食动物, 2003. 23(6):33-34. |
| Li YF. Progress in livestock sex control technology[J]. Chinese Herbivores, 2003. 23(6):33-34. | |
| [2] | 廖勤丰, 何航, 陈亚强, 等. 哺乳动物性别决定相关基因研究进展[J]. 家畜生态学报, 2019. 40(7):9-12. |
| Liao QF, He H, Chen YQ, et al. Advances in the study of genes related to sex determination in mammals[J]. Journal of Domestic Animal Ecology, 2019. 40(7):9-12. | |
| [3] |
Marei WFA, Khalil WA, Pushpakumara APG, et al. Polyunsaturated fatty acids influence offspring sex ratio in cows[J]. International Journal of Veterinary Science and Medicine, 2018. 6:S36-S40.
doi: 10.1016/j.ijvsm.2018.01.006 URL |
| [4] |
Song WH, Mohamed EA, Pang WK, et al. Effect of endocrine disruptors on the ratio of X and Y chromosome-bearing live spermatozoa[J]. Reproductive Toxicology, 2018. 82:10-17.
doi: 10.1016/j.reprotox.2018.09.002 URL |
| [5] | 赵红恩. 受精时阴道酸碱度变化对子代家兔性别的影响[J]. 中国优生与遗传杂志, 2015. 23(9):119. |
| Zhao HE. The effect of changes in vaginal pH during fertilization on the sex of offspring rabbits[J]. Chinese Journal of Health and Genetics, 2015. 23(9):119. | |
| [6] | 陆阳清, 张明, 卢克焕. 流式细胞仪分离精子法的研究进展[J]. 生物技术通报, 2005(3):26-30. |
| Lu YQ, Zhang M, Lu KH. Progress in flow cytometry for sperm isolation[J]. Biotechnology Bulletin, 2005(3):26-30. | |
| [7] |
Schenk JL, Suh TK, Cran DG, et al. Cryopreservation of flow-sorted bovine spermatozoa[J]. Theriogenology, 1999,52(8):1375-1391.
pmid: 10735083 |
| [8] | 王海浪, 薛建华, 吕小青, 等. 流式细胞仪生产牛性控冷冻精液技术要点[J]. 中国奶牛, 2014(1):53-55. |
| Wang HL, Xue JH, Lv XQ, et al. Key points of flow cytometry for bovine sex control and frozen semen production[J]. Chinese Dairy Cows, 2014(1):53-55. | |
| [9] |
Galizi R, Doyle LA, Menichelli M, et al. A synthetic sex ratio distortion system for the control of the human malaria mosquito[J]. Nature Communications, 2014. 5(1):3977.
doi: 10.1038/ncomms4977 URL |
| [10] |
Galizi R, Hammond A, Kyrou K, et al. A CRISPR-Cas9 sex-ratio distortion system for genetic control[J]. Scientific Reports, 2016. 6(1):31139.
doi: 10.1038/srep31139 URL |
| [11] | 杨晓峰. 利用 CRISPRCas9 系统打靶 Rbmy 基因对小鼠性别比例影响的初步研究[D]. 广州:华南农业大学, 2017. |
| Yang XF. Preliminary study on the effect of CRISPRCas9 targeting Rbmy gene on the sex ratio in mice[D]. Guangzhou:South China Agricultural University, 2017. | |
| [12] |
Gaj T, Gersbach CA, Barbas CF. ZFN, TALEN, CRISPR/Cas-based methods for genome engineering[J]. Trends in Biotechnology, 2013,31(7):397-405.
doi: 10.1016/j.tibtech.2013.04.004 URL |
| [13] | 邓倩云, 常雪莹, 王斯佳, 等. 利用 CRISPR 系统介导人类 mir-505 基因位点的编辑[J]. 现代生物医学进展, 2016,16(2):257-260. |
| Deng QY, Chang XY, Wang SJ, et al. CRISPR mediated editing of human mir-505 gene loci[J]. Progress in Modern Biomedicine, 2016,16(2):257-260. | |
| [14] | 吴金青, 梅瑰, 刘志国, 等. 应用 SSA 报告载体提高 ZFN 和 CRISPR/Cas9 对猪 IGF2 基因的打靶效率[J]. 遗传, 2015,37(1):55-62. |
| Wu JQ, Mei G, Liu ZG, et al. Application of SSA reporting vector to improve the targeting efficiency of ZFN and CRISPR/Cas9 on pig IGF2 gene[J]. Hereditas, 2015,37(1):55-62. | |
| [15] | 刘燕, 任卫红, 王绿娅, 等. CRISPR/Cas9 基因编辑系统中两种 gRNA 活性检测方法比较[J]. 心肺血管病杂志, 2016,35(7):563-568. |
| Liu Y, Ren WH, Wang LY, et al. Comparison of two gRNA activity detection methods in CRISPR/Cas9 gene editing system[J]. Journal of Cardiovascular and Pulmonary Diseases, 2016,35(7):563-568. | |
| [16] |
Zuo E, Huo X, Yao X, et al. CRISPR/Cas9-mediated targeted chromosome elimination[J]. Genome Biology, 2017,18(1):224.
doi: 10.1186/s13059-017-1354-4 URL |
| [17] | 严雨倩, 周平坤. 哺乳动物细胞 DNA 非同源末端连接及其生物学意义[J]. 医学分子生物学杂志, 2006,3(1):69-72. |
| Yan YQ, Zhou PK. Non-homologous terminal connections of mammalian cell DNA and their biological significance[J]. Journal of Medical Molecular Biology, 2006,3(1):69-72. | |
| [18] |
Burma S, Chen BPC, Chen DJ. Role of non-homologous end joining(NHEJ)in maintaining genomic integrity[J]. DNA Repair, 2006,5(9):1042-1048.
doi: 10.1016/j.dnarep.2006.05.026 URL |
| [19] |
Bergs JWJ, Krawczyk PM, Borovski T, et al. Inhibition of homologous recombination by hyperthermia shunts early double strand break repair to non-homologous end-joining[J]. DNA Repair, 2013,12(1):38-45.
doi: 10.1016/j.dnarep.2012.10.008 URL |
| [20] | 蒋璐蔓, 李嵩, 杨晓峰, 等. 靶向小鼠 Rbmy 基因的 CRISPR/Cas9 系统高效切割位点筛选及其表达载体构建[J]. 畜牧与兽医, 2019,51(12):1-8. |
| Jiang LM, Li S, Yang XF, et al. Screening and expression vector construction of CRISPR/Cas9 system targeting Rbmy gene in mice[J]. Anim Husban Veter Med, 2019,51(12):1-8. | |
| [21] | 朱新产, 王宝维. 胚胎发育合子型基因激活(ZGA)的调控机制[J]. 西北农林科技大学学报:自然科学版, 2000,23(2):91-97. |
| Zhu XC, Wang BW. Regulation mechanism of zygotic gene activation(ZGA)in embryonic development[J]. Journal of Northwest A & F University:Natural Science Edition, 2000,23(2):91-97. | |
| [22] | 刘红林, 汪河海, 范必勤, 等. 乙醇激活诱导小鼠孤雌胚的发育与核型分析[J]. 畜牧与兽医, 2000,32(1):7-8. |
| Liu HL, Wang HH, Fan BQ, et al. In vitro development and karyotypes of ethanol activated parthenogenetic embryos in mouse[J]. Anim Husbn Veter Med, 2000,32(1):7-8. |
| [1] | 陈小玲, 廖东庆, 黄尚飞, 陈英, 芦志龙, 陈东. 利用CRISPR/Cas9系统改造酿酒酵母的研究进展[J]. 生物技术通报, 2023, 39(8): 148-158. |
| [2] | 杨玉梅, 张坤晓. 应用CRISPR/Cas9技术建立ERK激酶相分离荧光探针定点整合的稳定细胞株[J]. 生物技术通报, 2023, 39(8): 159-164. |
| [3] | 施炜涛, 姚春鹏, 魏文康, 王蕾, 房元杰, 仝钰洁, 马晓姣, 蒋文, 张晓爱, 邵伟. 利用CRISPR/Cas9技术构建MDH2敲除细胞株及抗呕吐毒素效应研究[J]. 生物技术通报, 2023, 39(7): 307-315. |
| [4] | 刘晓燕, 祝振亮, 史广宇, 华梓宇, 杨晨, 张涌, 刘军. 乳腺生物反应器的表达优化策略[J]. 生物技术通报, 2023, 39(5): 77-91. |
| [5] | 程静雯, 曹磊, 张艳敏, 叶倩, 陈敏, 谭文松, 赵亮. CHO细胞多基因工程改造策略的建立及应用[J]. 生物技术通报, 2023, 39(2): 283-291. |
| [6] | 黄文莉, 李香香, 周炆婷, 罗莎, 姚维嘉, 马杰, 张芬, 沈钰森, 顾宏辉, 王建升, 孙勃. 利用CRISPR/Cas9技术靶向编辑青花菜BoZDS[J]. 生物技术通报, 2023, 39(2): 80-87. |
| [7] | 王兵, 赵会纳, 余婧, 陈杰, 骆梅, 雷波. 利用CRISPR/Cas9系统研究REVOLUTA参与烟草叶芽发育的调控[J]. 生物技术通报, 2023, 39(10): 197-208. |
| [8] | 李双喜, 华进联. 抗猪繁殖与呼吸障碍综合征基因编辑猪研究进展[J]. 生物技术通报, 2023, 39(10): 50-57. |
| [9] | 林蓉, 郑月萍, 徐雪珍, 李丹丹, 郑志富. 拟南芥ACOL8基因在乙烯合成与响应中的功能分析[J]. 生物技术通报, 2023, 39(1): 157-165. |
| [10] | 刘静静, 刘晓蕊, 李琳, 王盈, 杨海元, 戴一凡. 利用CRISPR/Cas9技术建立OXTR基因敲除猪胎儿成纤维细胞系[J]. 生物技术通报, 2022, 38(6): 272-278. |
| [11] | Olalekan Amoo, 胡利民, 翟云孤, 范楚川, 周永明. 利用基因编辑技术研究BRANCHED1参与油菜分枝过程的调控[J]. 生物技术通报, 2022, 38(4): 97-105. |
| [12] | 丁亚群, 丁宁, 谢深民, 黄梦娜, 张昱, 张勤, 姜力. Vps28基因敲除小鼠模型的构建及其对泌乳和免疫性状影响的研究[J]. 生物技术通报, 2022, 38(3): 164-172. |
| [13] | 燕炯, 冯晨毅, 高学坤, 许祥, 杨佳敏, 陈朝阳. 基于CRISPR/Cas9技术构建Plin1基因敲除小鼠模型及表型分析[J]. 生物技术通报, 2022, 38(3): 173-180. |
| [14] | 钟菁, 孙玲玲, 张姝, 蒙园, 支怡飞, 涂黎晴, 徐天鹏, 濮黎萍, 陆阳清. 应用CRISPR/Cas9技术敲除Mda5基因对新城疫及传染性法氏囊病毒复制的影响[J]. 生物技术通报, 2022, 38(11): 90-96. |
| [15] | 宗梅, 韩硕, 郭宁, 段蒙蒙, 刘凡, 王桂香. 利用真空渗透和CRISPR/Cas9系统获得非转基因菜薹突变体[J]. 生物技术通报, 2022, 38(10): 159-163. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||