








生物技术通报 ›› 2024, Vol. 40 ›› Issue (6): 290-298.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0006
闫欢欢1,2( ), 尚怡彤1,2, 王丽红1,2, 田学琴1,2, 廖海艳1,2, 曾斌4, 胡志宏1,2,3(
), 尚怡彤1,2, 王丽红1,2, 田学琴1,2, 廖海艳1,2, 曾斌4, 胡志宏1,2,3( )
)
收稿日期:2024-01-04
出版日期:2024-06-26
发布日期:2024-05-14
通讯作者:
胡志宏,男,博士,副教授,研究方向:微生物分子生物学;E-mail: huzhihong426@163.com作者简介:闫欢欢,女,硕士研究生,研究方向:微生物分子生物学;E-mail: huan89215@163.com
基金资助:
YAN Huan-huan1,2( ), SHANG Yi-tong1,2, WANG Li-hong1,2, TIAN Xue-qin1,2, LIAO Hai-yan1,2, ZENG Bin4, HU Zhi-hong1,2,3(
), SHANG Yi-tong1,2, WANG Li-hong1,2, TIAN Xue-qin1,2, LIAO Hai-yan1,2, ZENG Bin4, HU Zhi-hong1,2,3( )
)
Received:2024-01-04
Published:2024-06-26
Online:2024-05-14
摘要:
【目的】 通过米曲霉中异源表达虫草素合成关键基因,构建米曲霉工程菌株合成虫草素。【方法】 以米曲霉尿苷/尿嘧啶和组氨酸双营养缺陷型菌株AoΔpyrGΔHisB为背景菌株,利用农杆菌介导的转化方法对虫草素合成关键基因CmCns1-CmCns3进行过表达;通过荧光显微镜观察CmCns1和CmCns2在米曲霉中的亚细胞定位;利用高效液相色谱法(HPLC)测定转基因米曲霉虫草素含量,同时对米曲霉发酵液添加合成虫草素的前体甘氨酸和腺嘌呤,探究其在米曲霉中对合成虫草素的影响。【结果】 蛹虫草CmCns1和CmCns2在米曲霉中定位于脂滴;并且米曲霉中单独过表达CmCns1、共表达CmCns1和CmCns2以及共表达CmCns1-CmCns3均能合成虫草素,发酵48 h虫草素胞外产量最高达到37.74 μg/mL;向米曲霉发酵液添加甘氨酸和腺嘌呤,不能有效提升虫草素的含量。【结论】 成功在米曲霉异源表达合成虫草素。
闫欢欢, 尚怡彤, 王丽红, 田学琴, 廖海艳, 曾斌, 胡志宏. 米曲霉异源表达合成虫草素[J]. 生物技术通报, 2024, 40(6): 290-298.
YAN Huan-huan, SHANG Yi-tong, WANG Li-hong, TIAN Xue-qin, LIAO Hai-yan, ZENG Bin, HU Zhi-hong. Heterologous Biosynthesis of Cordycepin in Aspergillus oryzae[J]. Biotechnology Bulletin, 2024, 40(6): 290-298.
| Primer name | Forward primer(5'-3') | Reverse primer(5'-3') |
|---|---|---|
| Cns1-pEX1-GFP | GAGCAGACATCACCCTCGAGATGGCCATGAACGAGAACGC | ATGGTACCTACGTACTCGAGGGCTATGCCCACCTTGGATC |
| Cns2-pEX2D-DsRed | CACAGAAGGCATTTCACGTGATGTCTTGTCCTACCAGCGC | TCCTTAAGCACGGGCACGTGTCGATGCTGCGTGCGGCTC |
| Cns3-pEX1 | GAGCAGACATCACCCTCGAGATGTCCGAGTCAACCGCCTA | ATGGTACCTACGTACTCGAGCACACGCTGATAAAGGCCGA |
| Cns3-pEX1-BFP | CCCTCGAGTACGTAGGTACCATGGTGTCTAAGGGCGAAGA | CCCTTGCTCACCATGGTACCATTAAGCTTGTGCCCCAGTT |
| Cns1-GFP/Cns2-DsRed-pEX2D | TATGACATGATTACGAATTCTCAGAGCCTAGCCAACTAGT | CTCCAGATCGCAGCGAATTCCGACGGCCAGTGCCAAGCTT |
表1 构建载体所用引物
Table 1 Primers used for vector construction
| Primer name | Forward primer(5'-3') | Reverse primer(5'-3') |
|---|---|---|
| Cns1-pEX1-GFP | GAGCAGACATCACCCTCGAGATGGCCATGAACGAGAACGC | ATGGTACCTACGTACTCGAGGGCTATGCCCACCTTGGATC |
| Cns2-pEX2D-DsRed | CACAGAAGGCATTTCACGTGATGTCTTGTCCTACCAGCGC | TCCTTAAGCACGGGCACGTGTCGATGCTGCGTGCGGCTC |
| Cns3-pEX1 | GAGCAGACATCACCCTCGAGATGTCCGAGTCAACCGCCTA | ATGGTACCTACGTACTCGAGCACACGCTGATAAAGGCCGA |
| Cns3-pEX1-BFP | CCCTCGAGTACGTAGGTACCATGGTGTCTAAGGGCGAAGA | CCCTTGCTCACCATGGTACCATTAAGCTTGTGCCCCAGTT |
| Cns1-GFP/Cns2-DsRed-pEX2D | TATGACATGATTACGAATTCTCAGAGCCTAGCCAACTAGT | CTCCAGATCGCAGCGAATTCCGACGGCCAGTGCCAAGCTT |
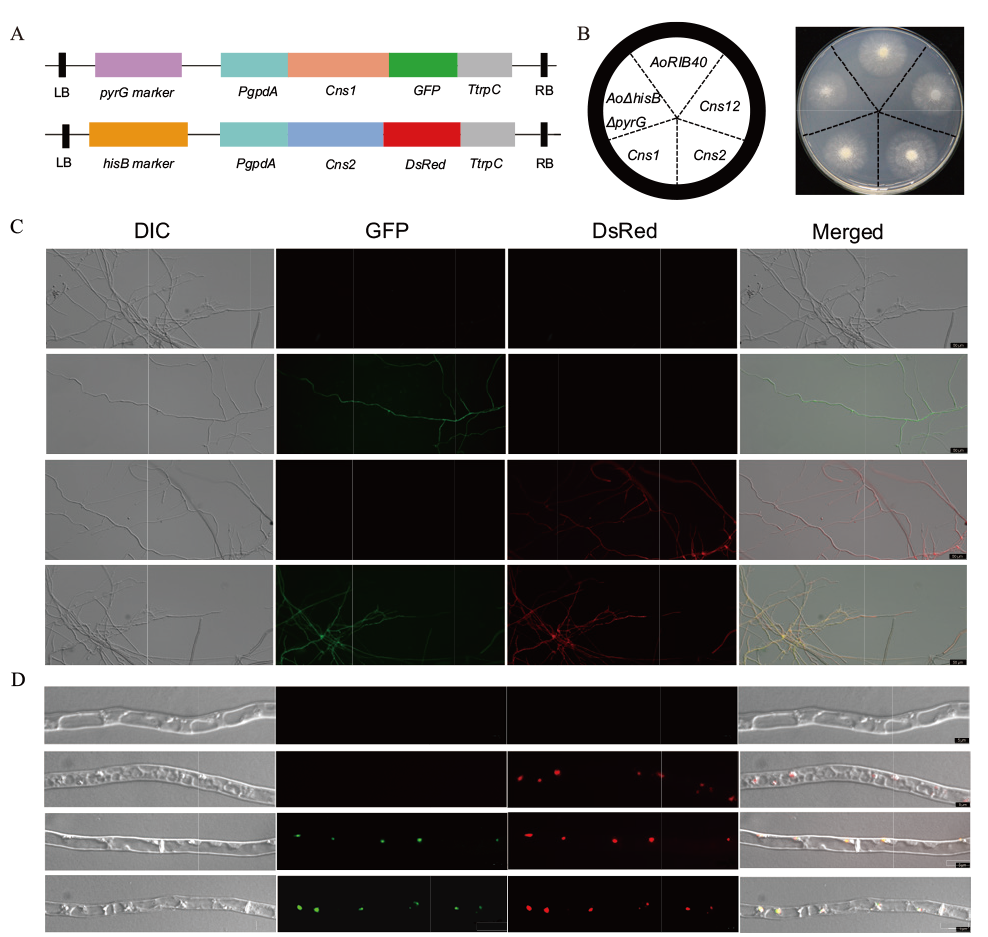
图1 CmCns1和CmCns2在米曲霉中亚细胞定位 A:Cns1-pEX1-GFP、Cns2-pEX2D-DsRed在米曲霉中过表达载体构建示意图;B:野生型AoRIB40、营养缺陷型菌株AoΔpyrGΔHisB、转化子CmCns1/AoΔpyrGΔHisB、CmCns2/AoΔpyrGΔHisB和CmCns1 CmCns2/AoΔpyrGΔHisB在CD+Uri+Ura+His培养3-5 d的表型;C:转化子荧光观察,从上到下依次是AoRIB40、转化子CmCns1/AoΔpyrGΔHisB、CmCns2/AoΔpyrGΔHisB和CmCns1 CmCns2/AoΔpyrGΔHisB在20倍物镜菌丝体的显微镜观察;D:CmCns1和CmCns2在米曲霉中的亚细胞定位,从上到下依次为AoRIB40、AoRIB40与尼罗红染料染色、转化子CmCns1/AoΔpyrGΔHisB与尼罗红染料染色、CmCns1 CmCns2/AoΔpyrGΔHisB在63倍镜下菌丝体的显微镜观察。C和D中从左至右:DIC,GFP绿色荧光,DsRed红色荧光,GFP、DsRed与DIC组合图像
Fig. 1 Subcellular localization of CmCns1 and CmCns2 in A. oryzae A: The scheme of Cns1-pEX1-GFP and Cns2-pEX2D-DsRed overexpression vectors in A. oryzae. B: Wild-type AoRIB40, nutrient deficient strain AoΔpyrGΔHisB, transformant CmCns1/AoΔpyrGΔHisB, CmCns2/AoΔpyrGΔHisB and CmCns1 CmCns2/AoΔpyrGΔHisB were phenotyped in CD+Uri+Ura+His medium at 30℃ for 3-5 d. C: Fluorescence observation of transformants, from top to bottom: microscopic observation of mycelium of AoRIB40, transformant CmCns1/AoΔpyrGΔHisB, CmCns2/AoΔpyrGΔHisB and CmCns1 CmCns2/AoΔpyrGΔHisB in a 20-fold objective. D: Subcellular localization of CmCns1 and CmCns2 in A. oryzae, from top to bottom: microscopic observation of mycelium with AoRIB40, AoRIB40 stained with Nile red dye, transformant of CmCns1/AoΔpyrGΔHisB stained with Nile red dye, and CmCns1 CmCns2/AoΔpyrGΔHisB under 63-fold microscope. C and D: From left to right: DIC, green fluorescence of GFP, red fluorescence of DsRed, and combined images of GFP, DsRed and DIC
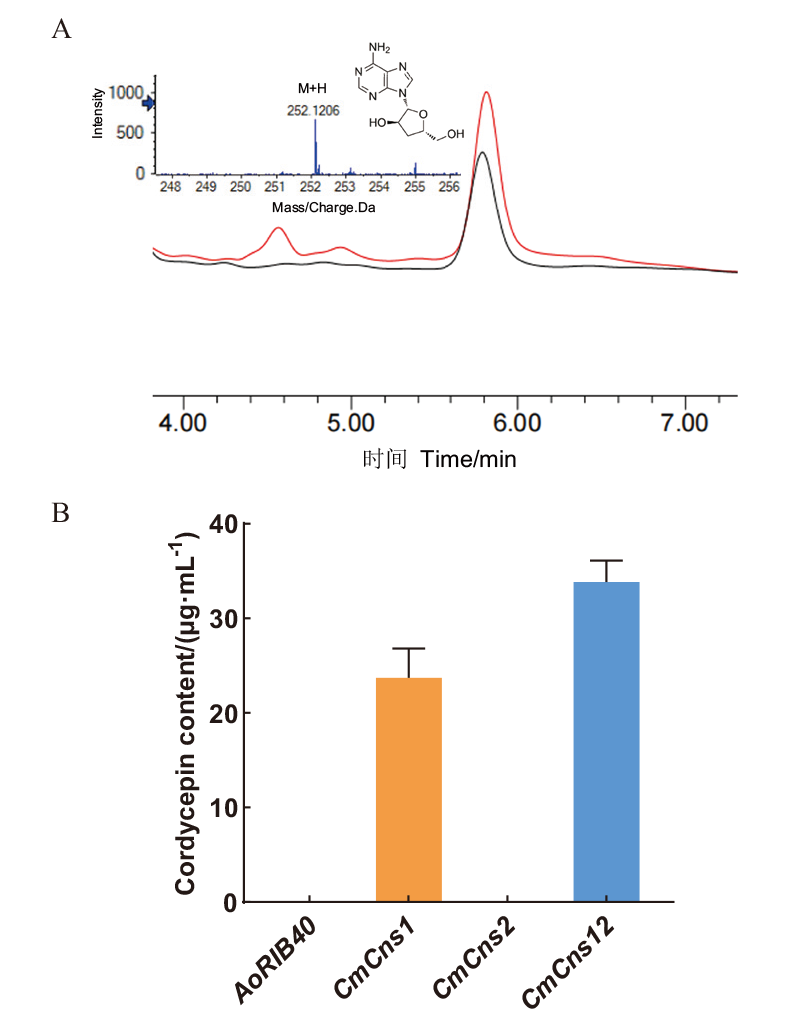
图2 米曲霉异源表达CmCns1和CmCns2虫草素含量 A:米曲霉转化子CmCns1/AoΔpyrGΔHisB和CmCns1 CmCns2/AoΔpyrGΔHisB虫草素的液相色谱峰及CmCns1 CmCns2/AoΔpyrGΔHisB的LC-MS/MS分析,黑色和红色峰形分别代表CmCns1/AoΔpyrGΔHisB和CmCns1 CmCns2/AoΔpyrGΔHisB;B:米曲霉对照AoRIB40、转化子CmCns1/AoΔpyrGΔHisB、CmCns2/AoΔpyrGΔHisB和CmCns1 CmCns2/AoΔpyrGΔHisB液体发酵培养基中虫草素的含量
Fig. 2 Cordycepin contents of CmCns1 and CmCns2 heterologously expressed in A. oryzae A: The chromatogram peaks of cordycepin in A. oryzae transformant CmCns1/AoΔpyrGΔHisB and CmCns1 CmCns2/AoΔpyrGΔHisB by HPLC, and LC-MS/MS analysis of CmCns1 CmCns2/AoΔpyrGΔHisB. The black and red peaks respectively indicate CmCns1/AoΔpyrGΔHisB and CmCns1 CmCns2/AoΔpyrGΔHisB.B: Cordycepin contents in A. oryzae AoRIB40, heterologous expressing transformant CmCns1/AoΔpyrGΔHisB, CmCns2/AoΔpyrGΔHisB and CmCns1 CmCns2/AoΔpyrGΔHisB in liquid fermentation medium

图3 米曲霉异源表达CmCns1、CmCns2和CmCns3虫草素含量 A:Cns1-GFP/Cns2-DsRed-pEX2D、Cns3-pEX1-BFP载体构建示意图;B:野生型米曲霉AoRIB40、背景菌株AoΔpyrGΔHisB、米曲霉转化子CmCns1 CmCns2-pEX2D/AoΔpyrGΔHisB、CmCns3/AoΔpyrGΔHisB和CmCns1 CmCns2 CmCns3/AoΔpyrGΔHisB在30℃使用CD+Uri+Ura+His培养3-5 d的表型;C:从上到下依次是米曲霉转化子CmCns1 CmCns2-pEX2D/AoΔpyrGΔHisB、CmCns3/AoΔpyrGΔHisB和CmCns1 CmCns2 CmCns3/AoΔpyrGΔHisB在40倍镜下菌丝体显微镜观察,从左至右依次是DIC,GFP绿色荧光,DsRed红色荧光,BFP蓝色荧光,GFP、DsRed、BFP三种荧光与DIC组合图像;D:米曲霉AoRIB40、异源表达转化子CmCns1/AoΔpyrGΔHisB、CmCns1 CmCns2/AoΔpyrGΔHisB和CmCns1 CmCns2 CmCns3/AoΔpyrGΔHisB液体发酵培养基中虫草素的含量;E:米曲霉转化子CmCns1/AoΔpyrGΔHisB、CmCns1 CmCns2/AoΔpyrGΔHisB和CmCns1 CmCns2 CmCns3/AoΔpyrGΔHisB虫草素的液相色谱峰,红色、蓝色和黑色峰形分别对应CmCns1/AoΔpyrGΔHisB、CmCns1 CmCns2/AoΔpyrGΔHisB和CmCns1 CmCns2 CmCns3/AoΔpyrGΔHisB。**表示与对照组相比有显著差异(P<0.01),下同
Fig. 3 Cordycepin contents of CmCns1, CmCns2 and CmCns3 heterologously expressed in A. oryzae A: The scheme of Cns1-GFP/Cns2-DsRed-pEX2D and Cns3-pEX1-BFP vectors. B: Wild-type A. oryzae AoRIB40, background strain AoΔpyrGΔHisB, transformant CmCns1 CmCns2-pEX2D/AoΔpyrGΔHisB, CmCns3/AoΔpyrGΔHisB and CmCns1 CmCns2 CmCns3/AoΔpyrGΔHisB were phenotyped in CD+Uri+Ura+His medium at 30℃ for 3-5 d.C: From top to bottom : Microscopic observation of mycelium of transformant CmCns1CmCns2-pEX2D/AoΔpyrGΔHisB, CmCns3/AoΔpyrGΔHisB and CmCns1 CmCns2 CmCns3/AoΔpyrGΔHisB in a 40-fold objective; from left to right: DIC, green fluorescence of GFP, red fluorescence of DsRed, blue fluorescence of BFP, combined images of GFP, DsRed, BFP and DIC. D: The contents of cordycepin in A. oryzae AoRIB40 and heterologous expression transformant CmCns1/AoΔpyrGΔHisB, CmCns1 CmCns2/AoΔpyrGΔHisB and CmCns1 CmCns2 CmCns3/AoΔpyrGΔHisB liquid fermentation medium. E: The chromatogram peaks of cordycepin contents in A. oryzae transformants CmCns1/AoΔpyrGΔHisB, CmCns1 CmCns2/AoΔpyrGΔHisB and CmCns1 CmCns2 CmCns3/AoΔpyrGΔHisB by HPLC, the red, blue, and black peaks respectively correspond to CmCns1/AoΔpyrGΔHisB, CmCns1 CmCns2/AoΔpyrGΔHisB and CmCns1 CmCns2 CmCns3/AoΔpyrGΔHisB. ** indicate a significant difference compared to the control(P<0.01), the same below
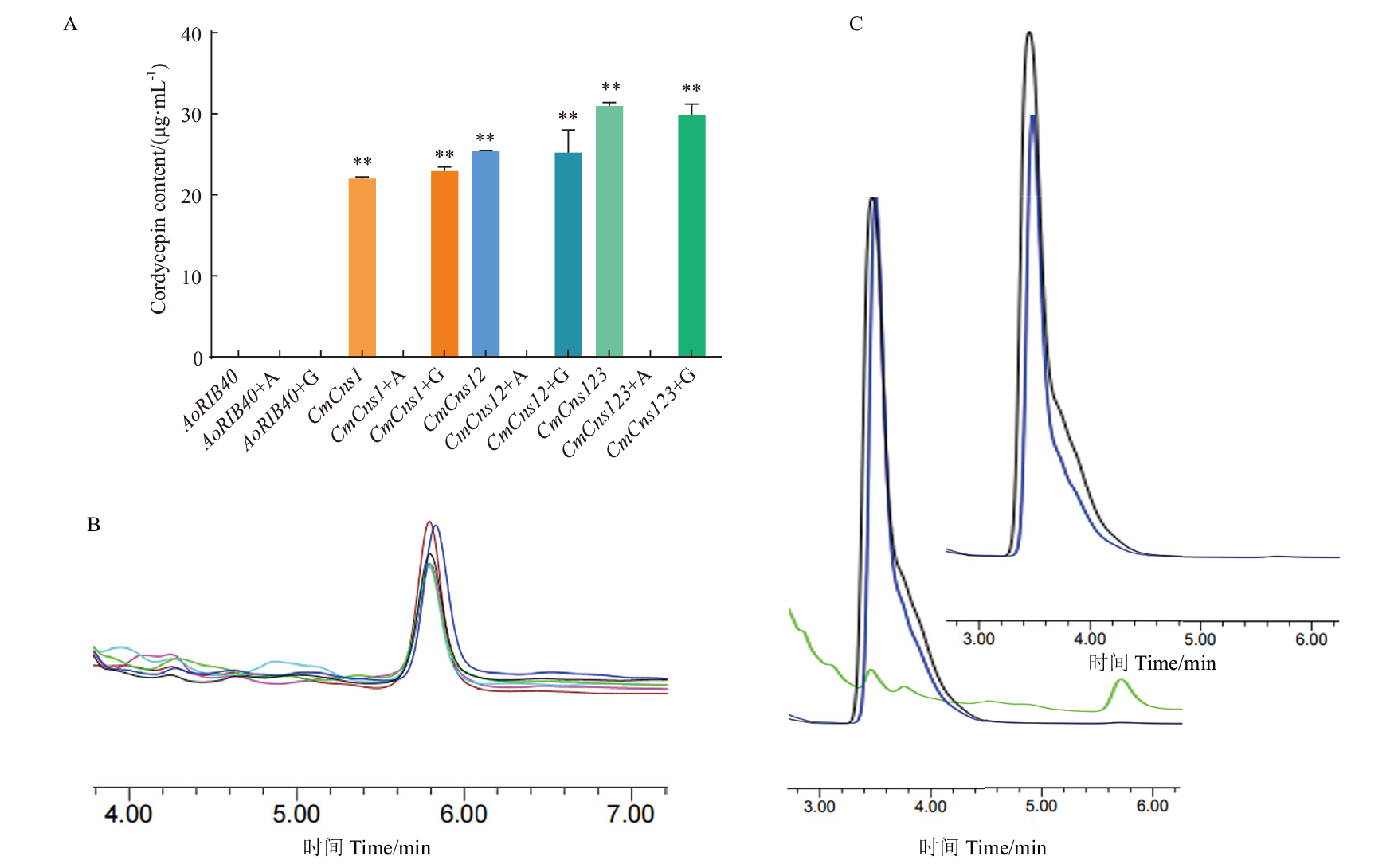
图4 米曲霉异源表达CmCns1、CmCns2和CmCns3液体发酵液添加腺嘌呤和甘氨酸虫草素含量 A:米曲霉AoRIB40、米曲霉转化子CmCns1/AoΔpyrGΔHisB、CmCns1 CmCns2/AoΔpyrGΔHisB和CmCns1 CmCns2 CmCns3/AoΔpyrGΔHisB在DPY培养基以及在培养基中分别添加甘氨酸和腺嘌呤的虫草素含量,AoRIB40+A代表向培养基中添加腺嘌呤,AoRIB40+G代表在培养基中添加甘氨酸,其余以此类推;B:米曲霉转化子CmCns1/AoΔpyrGΔHisB、CmCns1 CmCns2/AoΔpyrGΔHisB和CmCns1 CmCns2 CmCns3/AoΔpyrGΔHisB及添加甘氨酸后虫草素的液相色谱图,青色、红色、黑色、紫色、绿色和蓝色分别是CmCns1/AoΔpyrGΔHisB、CmCns1 CmCns2/AoΔpyrGΔHisB和CmCns1 CmCns2 CmCns3/AoΔpyrGΔHisB及添加甘氨酸后的峰形;C:米曲霉AoRIB40和转化子CmCns1/AoΔpyrGΔHisB添加腺嘌呤前以及添加后虫草素的液相色谱峰,黑色、蓝色和绿色峰形分别对应AoRIB40、CmCns1/AoΔpyrGΔHisB添加腺嘌呤后和CmCns1/AoΔpyrGΔHisB添加腺嘌呤前
Fig. 4 Cordycepin contents of CmCns1, CmCns2 and CmCns3 heterologously expressed in A. oryzae in the liquid fermentation broth with adenine and glycine A: Cordycepin contents of A. oryzae AoRIB40, transformant CmCns1/AoΔpyrGΔHisB, CmCns1 CmCns2/AoΔpyrGΔHisB and CmCns1CmCns2 CmCns3/AoΔpyrGΔHisB in DPY medium and with glycine and adenine in the medium, respectively, AoRIB40+A indicates the addition of adenine to the medium, AoRIB40+G indicates the addition of glycine to the medium, and so on. B: Cordycepin content plots of A. oryzae transformant CmCns1/AoΔpyrGΔHisB, CmCns1 CmCns2/AoΔpyrGΔHisB, CmCns1 CmCns2 CmCns3/AoΔpyrGΔHisB and addition of glycine to the medium by HPLC, cyan, red, black, purple, green, and blue are the peaks of CmCns1/AoΔpyrGΔHisB, CmCns1 CmCns2/AoΔpyrGΔHisB, CmCns1 CmCns2 CmCns3/AoΔpyrGΔHisB and the addition of glycine, respectively. C: The chromatogram peaks of cordycepin in AoRIB40 and transformant CmCns1/AoΔpyrGΔHisB by HPLC before and after addition of adenine, the black, blue and green peaks correspond to AoRIB40, CmCns1/AoΔpyrGΔHisB after adding adenine and CmCns1/AoΔpyrGΔHisB before adding adenine, respectively
| [1] | Yang LY, Li GL, Chai Z, et al. Synthesis of cordycepin: current scenario and future perspectives[J]. Fungal Genet Biol, 2020, 143: 103431. |
| [2] |
Jin Y, Meng X, Qiu ZD, et al. Anti-tumor and anti-metastatic roles of cordycepin, one bioactive compound of Cordyceps militaris[J]. Saudi J Biol Sci, 2018, 25(5): 991-995.
doi: 10.1016/j.sjbs.2018.05.016 pmid: 30108453 |
| [3] | Taghinejad Z, Kazemi T, Fadaee M, et al. Pharmacological and therapeutic potentials of cordycepin in hematological malignancies[J]. Biochem Biophys Res Commun, 2023, 678: 135-143. |
| [4] |
Khan MA, Tania M. Cordycepin in anticancer research: molecular mechanism of therapeutic effects[J]. Curr Med Chem, 2020, 27(6): 983-996.
doi: 10.2174/0929867325666181001105749 pmid: 30277143 |
| [5] | Wang L, Yan HH, Zeng B, et al. Research progress on cordycepin synthesis and methods for enhancement of cordycepin production in Cordyceps militaris[J]. Bioengineering, 2022, 9(2): 69. |
| [6] |
Xia YL, Luo FF, Shang YF, et al. Fungal cordycepin biosynthesis is coupled with the production of the safeguard molecule pentostatin[J]. Cell Chem Biol, 2017, 24(12): 1479-1489.e4.
doi: S2451-9456(17)30327-6 pmid: 29056419 |
| [7] |
张永杰, 张姝. 蛹虫草组学研究进展[J]. 菌物学报, 2021, 40(11): 2881-2893.
doi: 10.13346/j.mycosystema.210327 |
| Zhang YJ, Zhang S. Research progress on the Cordyceps militaris omics[J]. Mycosystema, 2021, 40(11): 2881-2893. | |
| [8] | 霍春红, 李鸿宇, 李倩, 等. 产虫草素酿酒酵母工程菌株的构建与发酵优化[J]. 生物工程学报, 2021, 37(9): 3334-3347. |
| Huo CH, Li HY, Li Q, et al. Construction and optimization of cordycepin-producing Saccharomyces cerevisiae[J]. Chin J Biotechnol, 2021, 37(9): 3334-3347. | |
| [9] | Song ZQ, Lin WB, Duan XY, et al. Increased cordycepin production in Yarrowia lipolytica using combinatorial metabolic engineering strategies[J]. ACS Synth Biol, 2023, 12(3): 780-787. |
| [10] | Yu JY, Sun M, Wang XY, et al. Poly-pathways metabolomics for high-yielding cordycepin of Cordyceps militaris[J]. Biomed Chromatogr, 2023, 37(2): e5551. |
| [11] | Masuda M, Urabe E, Honda H, et al. Enhanced production of cordycepin by surface culture using the medicinal mushroom Cordyceps militaris[J]. Enzyme Microb Technol, 2007, 40(5): 1199-1205. |
| [12] | Sari N, Suparmin A, Kato T, et al. Improved cordycepin production in a liquid surface culture of Cordyceps militaris isolated from wild strain[J]. Biotechnol Bioprocess Eng, 2016, 21(5): 595-600. |
| [13] | Machida M, Yamada O, Gomi K. Genomics of Aspergillus oryzae: learning from the history of Koji mold and exploration of its future[J]. DNA Res, 2008, 15(4): 173-183. |
| [14] | Wang L, Hu TT, Jiang ZQ, et al. Efficient production of a novel alkaline cold-active phospholipase C from Aspergillus oryzae by molecular chaperon co-expression for crude oil degumming[J]. Food Chem, 2021, 350: 129212. |
| [15] | Machida M, Asai K, Sano M, et al. Genome sequencing and analysis of Aspergillus oryzae[J]. Nature, 2005, 438(7071): 1157-1161. |
| [16] | Fujii R, Minami A, Tsukagoshi T, et al. Total biosynthesis of diterpene aphidicolin, a specific inhibitor of DNA polymerase α: heterologous expression of four biosynthetic genes in Aspergillus oryzae[J]. Biosci Biotechnol Biochem, 2011, 75(9): 1813-1817. |
| [17] | Ban A, Tanaka M, Fujii R, et al. Subcellular localization of aphidicolin biosynthetic enzymes heterologously expressed in Aspergillus oryzae[J]. Biosci Biotechnol Biochem, 2018, 82(1): 139-147. |
| [18] | Ugai T, Minami A, Fujii R, et al. Heterologous expression of highly reducing polyketide synthase involved in betaenone biosynthesis[J]. Chem Commun, 2015, 51(10): 1878-1881. |
| [19] | Tagami K, Minami A, Fujii R, et al. Rapid reconstitution of biosynthetic machinery for fungal metabolites in Aspergillus oryzae: total biosynthesis of aflatrem[J]. Chembiochem, 2014, 15(14): 2076-2080. |
| [20] | Feng J, Hauser M, Cox RJ, et al. Engineering Aspergillus oryzae for the heterologous expression of a bacterial modular polyketide synthase[J]. J Fungi, 2021, 7(12): 1085. |
| [21] | Thai HD, Nguyen BPT, Nguyen VM, et al. Development of a new Agrobacterium-mediated transformation system based on a dual auxotrophic approach in the filamentous fungus Aspergillus oryzae[J]. World J Microbiol Biotechnol, 2021, 37(6): 92. |
| [22] | Sun YL, Niu YL, Huang H, et al. Mevalonate diphosphate decarboxylase MVD/Erg19 is required for ergosterol biosynthesis, growth, sporulation and stress tolerance in Aspergillus oryzae[J]. Front Microbiol, 2019, 10: 1074. |
| [23] | Jin Q, Li GH, Qin KH, et al. The expression pattern, subcellular localization and function of three sterol 14α-demethylases in Aspergillus oryzae[J]. Front Genet, 2023, 14: 1009746. |
| [24] | Hu ZH, Huang H, Sun YL, et al. Effects on gene transcription profile and fatty acid composition by genetic modification of mevalonate diphosphate decarboxylase MVD/Erg19 in Aspergillus oryzae[J]. Microorganisms, 2019, 7(9): 342. |
| [25] | 闫欢欢, 尚怡彤, 王丽红, 等. 蛹虫草磷酸甲羟戊酸激酶和焦磷酸甲羟戊酸脱羧酶基因的功能分析[J]. 微生物学报, 2024, 64(2): 461-472. |
| Yan HH, Shang YT, Wang LH, et al. Functions of genes encoding phosphomevalonate kinase and mevalonate diphosphate decarboxylase in Cordyceps militaris[J]. Acta Microbiologica Sinica, 2024, 64(2): 461-472. | |
| [26] | Nguyen KT, Ho QN, Pham TH, et al. The construction and use of versatile binary vectors carrying pyrG auxotrophic marker and fluorescent reporter genes for Agrobacterium-mediated transformation of Aspergillus oryzae[J]. World J Microbiol Biotechnol, 2016, 32(12): 204. |
| [27] | 王立, 黄慧, 刘新平, 等. 农杆菌介导的蛹虫草营养缺陷型菌株和遗传转化体系的构建[J]. 微生物学通报, 2022, 49(8): 3373-3386. |
| Wang L, Huang H, Liu XP, et al. Construction of Agrobacterium-mediated auxotrophic strain and genetic transformation system of Cordyceps militaris[J]. Microbiol China, 2022, 49(8): 3373-3386. | |
| [28] | Chen M, Luo JH, Jiang WM, et al. Cordycepin: a review of strategies to improve the bioavailability and efficacy[J]. Phytother Res, 2023, 37(9): 3839-3858. |
| [29] | Turk A, Abdelhamid MAA, Yeon SW, et al. Cordyceps mushroom with increased cordycepin content by the cultivation on edible insects[J]. Front Microbiol, 2022, 13: 1017576. |
| [30] | Turk A, Lee S, Yeon SW, et al. Adenosine deaminase inhibitory activity of medicinal plants: boost the production of cordycepin in Cordyceps militaris[J]. Antioxidants, 2023, 12(6): 1260. |
| [31] | Duan XY, Yang H, Wang C, et al. Microbial synthesis of cordycepin, current systems and future perspectives[J]. Trends Food Sci Technol, 2023, 132: 162-170. |
| [32] | Borde M, Singh SK. Enhanced production of cordycepin under solid-state fermentation of Cordyceps militaris by using combinations of grains/substrates[J]. Braz J Microbiol, 2023, 54(4): 2765-2772. |
| [33] |
Alberti F, Foster GD, Bailey AM. Natural products from filamentous fungi and production by heterologous expression[J]. Appl Microbiol Biotechnol, 2017, 101(2): 493-500.
doi: 10.1007/s00253-016-8034-2 pmid: 27966047 |
| [1] | 李博静, 郑腊梅, 吴乌云, 高飞, 周宜君. 西蒙得木HSP20基因家族的进化、表达和功能分析[J]. 生物技术通报, 2024, 40(6): 190-202. |
| [2] | 吴泽航, 杨中义, 鄢毅铖, 贾永红, 吴月燕, 谢晓鸿. 比利时杜鹃花类黄酮3'-羟化酶(F3'H)基因克隆及功能分析[J]. 生物技术通报, 2024, 40(6): 251-259. |
| [3] | 李梦然, 叶伟, 李赛妮, 张维阳, 李建军, 章卫民. Lithocarols类化合物生物合成基因litI的表达及其启动子功能分析[J]. 生物技术通报, 2024, 40(6): 310-318. |
| [4] | 潘萍萍, 徐志浩, 张怡雯, 李青, 王忠华. 多花黄精查尔酮合酶PcCHS的原核表达、亚细胞定位及表达分析[J]. 生物技术通报, 2024, 40(5): 280-289. |
| [5] | 张震, 李清, 徐菁, 陈凯园, 张春芝, 祝光涛. 马铃薯线粒体靶向表达载体的构建与应用[J]. 生物技术通报, 2024, 40(5): 66-73. |
| [6] | 殷亮, 王代玮, 刘悦莹, 刘海燕, 罗光宏. 蛋白酶SpP1基因克隆、表达及酶学性质的表征[J]. 生物技术通报, 2024, 40(4): 278-286. |
| [7] | 杨艳, 胡洋, 刘霓如, 殷璐, 杨锐, 王鹏飞, 穆霄鹏, 张帅, 程春振, 张建成. ‘红满堂’苹果MbbZIP43基因的克隆与功能研究[J]. 生物技术通报, 2024, 40(2): 146-159. |
| [8] | 李昊, 伍国强, 魏明, 韩悦欣. 甜菜BvBADH基因家族全基因组鉴定及其高盐胁迫下的表达分析[J]. 生物技术通报, 2024, 40(2): 233-244. |
| [9] | 郑菲, 杨俊钊, 牛羽丰, 李蕊麟, 赵国柱. 嗜热毁丝菌裂解性多糖单加氧酶TtLPMO9I的酶学性质及其功能研究[J]. 生物技术通报, 2024, 40(2): 289-299. |
| [10] | 朱毅, 柳唐镜, 宫国义, 张洁, 王晋芳, 张海英. 西瓜ClPP2C3克隆及表达分析[J]. 生物技术通报, 2024, 40(1): 243-249. |
| [11] | 谢宏, 周丽莹, 李舒文, 王梦迪, 艾晔, 晁跃辉. 蒺藜苜蓿MtCIM基因结构和功能分析[J]. 生物技术通报, 2024, 40(1): 262-269. |
| [12] | 吕秋谕, 孙培媛, 冉彬, 王佳蕊, 陈庆富, 李洪有. 苦荞转录因子基因FtbHLH3的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 194-203. |
| [13] | 李博, 刘合霞, 陈宇玲, 周兴文, 朱宇林. 金花茶CnbHLH79转录因子的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 241-250. |
| [14] | 王帅, 冯宇梅, 白苗, 杜维俊, 岳爱琴. 大豆GmHMGR基因响应外源激素及非生物胁迫功能研究[J]. 生物技术通报, 2023, 39(7): 131-142. |
| [15] | 马玉倩, 孙东辉, 岳浩峰, 辛佳瑜, 刘宁, 曹志艳. 具有辅助降解纤维素功能的大斑刚毛座腔菌糖苷水解酶GH61的鉴定、异源表达及功能分析[J]. 生物技术通报, 2023, 39(4): 124-135. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||