








生物技术通报 ›› 2023, Vol. 39 ›› Issue (8): 194-203.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0034
吕秋谕( ), 孙培媛, 冉彬, 王佳蕊, 陈庆富, 李洪有(
), 孙培媛, 冉彬, 王佳蕊, 陈庆富, 李洪有( )
)
收稿日期:2023-01-16
出版日期:2023-08-26
发布日期:2023-09-05
通讯作者:
李洪有,男,博士,教授,研究方向:植物分子生物学;E-mail: lihongyouluod@163.com作者简介:吕秋谕,女,硕士,研究方向:植物分子生物学;E-mail: lqyswu@163.com
基金资助:
LYU Qiu-yu( ), SUN Pei-yuan, RAN Bin, WANG Jia-rui, CHEN Qing-fu, LI Hong-you(
), SUN Pei-yuan, RAN Bin, WANG Jia-rui, CHEN Qing-fu, LI Hong-you( )
)
Received:2023-01-16
Published:2023-08-26
Online:2023-09-05
摘要:
bHLH(basic helix-loop-helix)转录因子在植物黄酮化合物生物合成中具有重要的调控作用。为探究苦荞[Fagopyrum tataricum(L.)Gaertn]中bHLH转录因子在黄酮化合物生物合成中的功能和分子调控机制,利用RT-PCR(reverse transcription-polymerase chain reaction)技术从苦荞中克隆了一个bHLH转录因子基因FtbHLH3,并对其进行生物信息学、亚细胞定位、转录激活活性、基因表达、基因共表达和基因表达量与总黄酮含量相关性分析。结果表明,FtbHLH3全长CDS序列为783 bp,编码260个氨基酸。保守结构域和系统进化分析表明,FtbHLH3是一个非IIIf亚家族bHLH转录因子成员。亚细胞定位和转录激活活性分析显示,FtbHLH3定位于细胞核,不具有转录激活活性。基因共表达分析表明,FtbHLH3与苦荞中拟南芥TT8同源基因FtTT8和12个黄酮化合物生物合成结构基因共表达。不同组织部位中基因表达量与总黄酮含量间相关性分析表明,FtbHLH3的表达量与总黄酮含量间具有较高的正相关性。本研究结果表明,FtbHLH3是一个定位于细胞核,不具有转录激活活性的非IIIf亚家族bHLH转录因子,可能通过与其他转录因子互作来调控苦荞黄酮化合物的生物合成。
吕秋谕, 孙培媛, 冉彬, 王佳蕊, 陈庆富, 李洪有. 苦荞转录因子基因FtbHLH3的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 194-203.
LYU Qiu-yu, SUN Pei-yuan, RAN Bin, WANG Jia-rui, CHEN Qing-fu, LI Hong-you. Cloning, Subcellular Localization and Expression Analysis of the Transcription Factor Gene FtbHLH3 in Fagopyrum tataricum[J]. Biotechnology Bulletin, 2023, 39(8): 194-203.
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') |
|---|---|
| FtbHLH3F1 | ATGGATAACATCGGTGACGAGTAC |
| FtbHLH3R1 | TCAATTGATCGTAGCGCTATGAGG |
| FtbHLH3F2 | CAAGCTTGCATGCCTGCAGGTCGACATGGATAAC- ATCGGTGACGAG |
| FtbHLH3R2 | CCTCGCCCTTGCTCACCATGGATCCATTGATCGT- AGCGCTATGAG |
| FtbHLH3F3 | CCGGAATTCATGGATAACATCGGTGACGAGTAC |
| FtbHLH3R3 | CGCGGATCCTCAATTGATCGTAGCGCTATGAGG |
| FtbHLH3qF | GGTGTTTGAATCACTGGAGC |
| FtbHLH3qR | TCGTAGCGCTATGAGGTTGA |
| FtActin7F | ATGTTCACTACCACCGCTGA |
| FtActin7R | TGAACCTCTCAGCACCAATC |
表1 本研究所用引物序列
Table 1 Primer sequences used in the study
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') |
|---|---|
| FtbHLH3F1 | ATGGATAACATCGGTGACGAGTAC |
| FtbHLH3R1 | TCAATTGATCGTAGCGCTATGAGG |
| FtbHLH3F2 | CAAGCTTGCATGCCTGCAGGTCGACATGGATAAC- ATCGGTGACGAG |
| FtbHLH3R2 | CCTCGCCCTTGCTCACCATGGATCCATTGATCGT- AGCGCTATGAG |
| FtbHLH3F3 | CCGGAATTCATGGATAACATCGGTGACGAGTAC |
| FtbHLH3R3 | CGCGGATCCTCAATTGATCGTAGCGCTATGAGG |
| FtbHLH3qF | GGTGTTTGAATCACTGGAGC |
| FtbHLH3qR | TCGTAGCGCTATGAGGTTGA |
| FtActin7F | ATGTTCACTACCACCGCTGA |
| FtActin7R | TGAACCTCTCAGCACCAATC |

图5 FtbHLH3与苦荞种子差异表达bHLH转录因子基因和类黄酮生物合成结构基因的表达聚类热图 种子1:授粉后7 d的种子;种子2:授粉后13 d的种子;种子3:授粉后16 d的种子;-1和-2表示生物学重复1和2。红色框表示的是与bHLH3共表达的苦荞黄酮生物合成结构基因和bHLH转录因子基因
Fig. 5 Expression cluster heat map of FtbHLH3, differentially expressed transcription factor bHLH genes and flavonoid biosynthesis structure genes in tartary buckwheat seeds Seed 1: Seeds 7 d after pollination. Seed 2: Seeds 13 d after pollination. Seed 3: Seeds 16 d after pollination. - 1 and - 2 represent biological repeat 1 and 2. The red box shows the flavonoid biosynthesis structure genes and bHLH transcription factor genes co-expressed with bHLH3 of tartary buckwheat
| 基因名称 Gene name | FtbHLH3 | FtbHLH14 | FtbHLH29 | FtbHLH33/FtTT8 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |||||
| FtCHS-1 | 0.948 4 | 0.032 7 | 0.640 4 | 0.028 5 | 0.722 8 | 0.046 4 | 0.898 6 | 0.045 8 | ||||
| FtCHS-2 | 0.961 4 | 0.002 5 | 0.746 7 | 0.002 1 | 0.746 1 | 0.004 2 | 0.930 4 | 0.004 1 | ||||
| FtCHS-3 | 0.760 0 | 0.000 5 | 0.615 4 | 0.000 4 | 0.911 9 | 0.001 0 | 0.947 5 | 0.001 0 | ||||
| FtCHI | 0.890 8 | 0.001 7 | 0.752 6 | 0.000 9 | 0.833 7 | 0.011 1 | 0.975 7 | 0.008 4 | ||||
| FtF3H-1 | 0.849 8 | 0.000 2 | 0.825 2 | 0.000 2 | 0.676 1 | 0.000 3 | 0.880 3 | 0.000 3 | ||||
| FtF3H-2 | 0.522 4 | 3.15E-05 | 0.589 3 | 2.1E-05 | 0.701 6 | 0.000 1 | 0.798 4 | 8.19E-05 | ||||
| FtF3'H-1 | 0.810 2 | 0.001 0 | 0.595 8 | 0.000 7 | 0.929 6 | 0.002 6 | 0.976 7 | 0.002 3 | ||||
| FtF3'H-2 | 0.859 4 | 0.000 2 | 0.825 1 | 0.000 2 | 0.678 3 | 0.000 3 | 0.884 2 | 0.000 3 | ||||
| FtF3'5'H | 0.631 9 | 0.048 0 | 0.257 7 | 0.019 5 | 0.528 5 | 0.301 8 | 0.719 8 | 0.262 8 | ||||
| FtFLS | -0.239 6 | 6.36E-05 | 0.179 1 | 5.78E-05 | 0.144 7 | 8.18E-05 | 0.042 0 | 8.05E-05 | ||||
| FtDFR | 0.946 4 | 0.002 4 | 0.786 5 | 0.002 0 | 0.694 9 | 0.004 0 | 0.937 0 | 0.003 9 | ||||
| FtANS | 0.700 0 | 0.000 1 | 0.747 0 | 0.000 1 | 0.760 8 | 0.000 2 | 0.852 2 | 0.000 2 | ||||
| FtANR | 0.955 6 | 0.006 9 | 0.921 1 | 0.006 0 | 0.476 9 | 0.010 3 | 0.773 4 | 0.010 1 | ||||
| FtLAR | 0.923 6 | 0.014 7 | 0.782 2 | 0.011 4 | 0.754 4 | 0.028 1 | 0.947 8 | 0.026 9 | ||||
表2 FtbHLH3与类黄酮生物合成结构基因表达相关系数
Table 2 Correlation coefficient between FtbHLH3 and flavonoid biosynthesis structure gene expression
| 基因名称 Gene name | FtbHLH3 | FtbHLH14 | FtbHLH29 | FtbHLH33/FtTT8 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |||||
| FtCHS-1 | 0.948 4 | 0.032 7 | 0.640 4 | 0.028 5 | 0.722 8 | 0.046 4 | 0.898 6 | 0.045 8 | ||||
| FtCHS-2 | 0.961 4 | 0.002 5 | 0.746 7 | 0.002 1 | 0.746 1 | 0.004 2 | 0.930 4 | 0.004 1 | ||||
| FtCHS-3 | 0.760 0 | 0.000 5 | 0.615 4 | 0.000 4 | 0.911 9 | 0.001 0 | 0.947 5 | 0.001 0 | ||||
| FtCHI | 0.890 8 | 0.001 7 | 0.752 6 | 0.000 9 | 0.833 7 | 0.011 1 | 0.975 7 | 0.008 4 | ||||
| FtF3H-1 | 0.849 8 | 0.000 2 | 0.825 2 | 0.000 2 | 0.676 1 | 0.000 3 | 0.880 3 | 0.000 3 | ||||
| FtF3H-2 | 0.522 4 | 3.15E-05 | 0.589 3 | 2.1E-05 | 0.701 6 | 0.000 1 | 0.798 4 | 8.19E-05 | ||||
| FtF3'H-1 | 0.810 2 | 0.001 0 | 0.595 8 | 0.000 7 | 0.929 6 | 0.002 6 | 0.976 7 | 0.002 3 | ||||
| FtF3'H-2 | 0.859 4 | 0.000 2 | 0.825 1 | 0.000 2 | 0.678 3 | 0.000 3 | 0.884 2 | 0.000 3 | ||||
| FtF3'5'H | 0.631 9 | 0.048 0 | 0.257 7 | 0.019 5 | 0.528 5 | 0.301 8 | 0.719 8 | 0.262 8 | ||||
| FtFLS | -0.239 6 | 6.36E-05 | 0.179 1 | 5.78E-05 | 0.144 7 | 8.18E-05 | 0.042 0 | 8.05E-05 | ||||
| FtDFR | 0.946 4 | 0.002 4 | 0.786 5 | 0.002 0 | 0.694 9 | 0.004 0 | 0.937 0 | 0.003 9 | ||||
| FtANS | 0.700 0 | 0.000 1 | 0.747 0 | 0.000 1 | 0.760 8 | 0.000 2 | 0.852 2 | 0.000 2 | ||||
| FtANR | 0.955 6 | 0.006 9 | 0.921 1 | 0.006 0 | 0.476 9 | 0.010 3 | 0.773 4 | 0.010 1 | ||||
| FtLAR | 0.923 6 | 0.014 7 | 0.782 2 | 0.011 4 | 0.754 4 | 0.028 1 | 0.947 8 | 0.026 9 | ||||
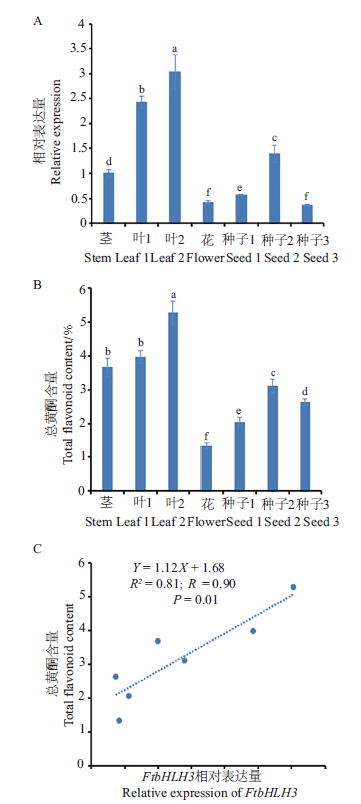
图6 FtbHLH3在苦荞不同组织部位中的表达量与总黄酮含量间的相关性
Fig. 6 Correlation between the expressions of FtbHLH3 in different tissue parts of tartary buckwheat and the contents of total flavonoids
| [1] |
Zhang KX, He M, Fan Y, et al. Resequencing of global Tartary buckwheat accessions reveals multiple domestication events and key loci associated with agronomic traits[J]. Genome Biol, 2021, 22(1): 23.
doi: 10.1186/s13059-020-02217-7 pmid: 33430931 |
| [2] |
Li HY, Lyu QY, Liu AK, et al. Comparative metabolomics study of Tartary(Fagopyrum tataricum(L.)Gaertn)and common(Fagop-yrum esculentum Moench)buckwheat seeds[J]. Food Chem, 2022, 371: 131125.
doi: 10.1016/j.foodchem.2021.131125 URL |
| [3] |
Xu WJ, Dubos C, Lepiniec L. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes[J]. Trends Plant Sci, 2015, 20(3): 176-185.
doi: 10.1016/j.tplants.2014.12.001 pmid: 25577424 |
| [4] |
高国应, 伍小方, 张大为, 等. MBW复合体在植物花青素合成途径中的研究进展[J]. 生物技术通报, 2020, 36(1): 126-134.
doi: 10.13560/j.cnki.biotech.bull.1985.2019-0738 |
| Gao GY, Wu XF, Zhang DW, et al. Research progress on the MBW complexes in plant anthocyanin biosynthesis pathway[J]. Biotechnol Bull, 2020, 36(1): 126-134. | |
| [5] |
Chandler VL, Radicella JP, Robbins TP, et al. Two regulatory genes of the maize anthocyanin pathway are homologous: isolation of B utilizing R genomic sequences[J]. Plant Cell, 1989, 1(12): 1175-1183.
pmid: 2535537 |
| [6] | Zhang BP, Schrader A. TRANSPARENT TESTA GLABRA 1-dependent regulation of flavonoid biosynthesis[J]. Plants(Basel), 2017, 6(4): 65. |
| [7] |
Escaray FJ, Passeri V, Perea-García A, et al. The R2R3-MYB TT2b and the bHLH TT8 genes are the major regulators of proanthocyanidin biosynthesis in the leaves of Lotus species[J]. Planta, 2017, 246(2): 243-261.
doi: 10.1007/s00425-017-2696-6 URL |
| [8] |
Lim SH, Kim DH, Kim JK, et al. A radish basic helix-loop-helix transcription factor, RsTT8 acts a positive regulator for anthocyanin biosynthesis[J]. Front Plant Sci, 2017, 8: 1917.
doi: 10.3389/fpls.2017.01917 URL |
| [9] |
Li CH, Qiu J, Ding L, et al. Anthocyanin biosynthesis regulation of DhMYB2 and DhbHLH1 in Dendrobium hybrids petals[J]. Plant Physiol Biochem, 2017, 112: 335-345.
doi: 10.1016/j.plaphy.2017.01.019 URL |
| [10] |
Zhao Y, Zhang YY, Liu H, et al. Functional characterization of a liverworts bHLH transcription factor involved in the regulation of bisbibenzyls and flavonoids biosynthesis[J]. BMC Plant Biol, 2019, 19(1): 497.
doi: 10.1186/s12870-019-2109-z pmid: 31726984 |
| [11] |
Heendeniya RG, Gruber MY, Lei Y, et al. Biodegradation profiles of proanthocyanidin-accumulating alfalfa plants coexpressing Lc-bHLH and C1-MYB transcriptive flavanoid regulatory genes[J]. J Agric Food Chem, 2019, 67(17): 4793-4799.
doi: 10.1021/acs.jafc.9b00495 URL |
| [12] |
Wang LH, Tang W, Hu YW, et al. A MYB/bHLH complex regulates tissue-specific anthocyanin biosynthesis in the inner pericarpof red-centered kiwifruit Actinidia chinensis cv. Hongyang[J]. Plant J, 2019, 99(2): 359-378.
doi: 10.1111/tpj.2019.99.issue-2 URL |
| [13] |
Su WQ, Tao R, Liu WY, et al. Characterization of four polymorphic genes controlling red leaf colour in lettuce that have undergone disruptive selection since domestication[J]. Plant Biotechnol J, 2020, 18(2): 479-490.
doi: 10.1111/pbi.13213 pmid: 31325407 |
| [14] |
Tao RY, Yu WJ, Gao YH, et al. Light-induced basic/helix-loop-helix64 enhances anthocyanin biosynthesis and undergoes CONSTITUTIVELY PHOTOMORPHOGENIC1-mediated degradation in pear[J]. Plant Physiol, 2020, 184(4): 1684-1701.
doi: 10.1104/pp.20.01188 pmid: 33093233 |
| [15] |
Zhao PC, Li XX, Jia JT, et al. bHLH92 from sheepgrass acts as a negative regulator of anthocyanin/proanthocyandin accumulation and influences seed dormancy[J]. J Exp Bot, 2019, 70(1): 269-284.
doi: 10.1093/jxb/ery335 pmid: 30239820 |
| [16] |
Burr FA, Burr B, Scheffler BE, et al. The maize repressor-like gene intensifier1 shares homology with the r1/b1 multigene family of transcription factors and exhibits missplicing[J]. Plant Cell, 1996, 8(8): 1249-1259.
pmid: 8776895 |
| [17] |
Liu XF, Yin XR, Allan AC, et al. The role of MrbHLH1 and MrMYB1 in regulating anthocyanin biosynthetic genes in tobacco and Chinese bayberry(Myrica rubra)during anthocyanin biosynthesis[J]. Plant Cell Tiss Organ Cult, 2013, 115(3): 285-298.
doi: 10.1007/s11240-013-0361-8 URL |
| [18] |
Hichri I, Barrieu F, Bogs J, et al. Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway[J]. J Exp Bot, 2011, 62(8): 2465-2483.
doi: 10.1093/jxb/erq442 pmid: 21278228 |
| [19] |
Bai YC, Li CL, Zhang JW, et al. Characterization of two Tartary buckwheat R2R3-MYB transcription factors and their regulation of proanthocyanidin biosynthesis[J]. Physiol Plant, 2014, 152(3): 431-440.
doi: 10.1111/ppl.2014.152.issue-3 URL |
| [20] |
Luo XP, Zhao HX, Yao PF, et al. An R2R3-MYB transcription factor FtMYB15 involved in the synthesis of anthocyanin and proanthocyanidins from Tartary buckwheat[J]. J Plant Growth Regul, 2018, 37(1): 76-84.
doi: 10.1007/s00344-017-9709-3 URL |
| [21] |
Huang YJ, Wu Q, Wang S, et al. FtMYB8 from Tartary buckwheat inhibits both anthocyanin/proanthocyanidin accumulation and marginal Trichome initiation[J]. BMC Plant Biol, 2019, 19(1): 263.
doi: 10.1186/s12870-019-1876-x pmid: 31215400 |
| [22] |
Dong QX, Zhao HX, Huang YJ, et al. FtMYB18 acts as a negative regulator of anthocyanin/proanthocyanidin biosynthesis in Tartary buckwheat[J]. Plant Mol Biol, 2020, 104(3): 309-325.
doi: 10.1007/s11103-020-01044-5 pmid: 32833148 |
| [23] |
Wang L, Deng RY, Bai YC, et al. Tartary buckwheat R2R3-MYB gene FtMYB3 negatively regulates anthocyanin and proanthocyanin biosynthesis[J]. Int J Mol Sci, 2022, 23(5): 2775.
doi: 10.3390/ijms23052775 URL |
| [24] |
Zhang D, Jiang CL, Huang CH, et al. The light-induced transcription factor FtMYB116 promotes accumulation of rutin in Fagopyrum tataricum[J]. Plant Cell Environ, 2019, 42(4): 1340-1351.
doi: 10.1111/pce.13470 |
| [25] |
Yao PF, Huang YJ, Dong QX, et al. FtMYB6, a light-induced SG7 R2R3-MYB transcription factor, promotes flavonol biosynthesis in Tartary buckwheat(Fagopyrum tataricum)[J]. J Agric Food Chem, 2020, 68(47): 13685-13696.
doi: 10.1021/acs.jafc.0c03037 URL |
| [26] |
Sun ZX, Bin LH, Hou SY, et al. Tartary buckwheat FtMYB31 gene encoding an R2R3-MYB transcription factor enhances flavonoid accumulation in tobacco[J]. J Plant Growth Regul, 2020, 39(2): 564-574.
doi: 10.1007/s00344-019-10000-7 |
| [27] |
Hou SY, Du W, Hao YR, et al. Elucidation of the regulatory network of flavonoid biosynthesis by profiling the metabolome and transcriptome in Tartary buckwheat[J]. J Agric Food Chem, 2021, 69(25): 7218-7229.
doi: 10.1021/acs.jafc.1c00190 URL |
| [28] |
孙小倩, 王佳蕊, 陈庆富, 等. 苦荞转录因子FtMYBF的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2021, 37(3): 10-17.
doi: 10.13560/j.cnki.biotech.bull.1985.2020-0861 |
| Sun XQ, Wang JR, Chen QF, et al. Gene cloning, subcellular localization and expression analyses of FtMYBF transcription factor in Fagopyrum tataricum[J]. Biotechnol Bull, 2021, 37(3): 10-17. | |
| [29] |
Zhou ML, Sun ZM, Ding MQ, et al. FtSAD2 and FtJAZ1 regulate activity of the FtMYB11 transcription repressor of the phenylpropanoid pathway in Fagopyrum tataricum[J]. New Phytol, 2017, 216(3): 814-828.
doi: 10.1111/nph.2017.216.issue-3 URL |
| [30] |
Zhang KX, Logacheva MD, Meng Y, et al. Jasmonate-responsive MYB factors spatially repress rutin biosynthesis in Fagopyrum tataricum[J]. J Exp Bot, 2018, 69(8): 1955-1966.
doi: 10.1093/jxb/ery032 URL |
| [31] |
Li JB, Zhang KX, Meng Y, et al. FtMYB16 interacts with Ftimportin-α1 to regulate rutin biosynthesis in Tartary buckwheat[J]. Plant Biotechnol J, 2019, 17(8): 1479-1481.
doi: 10.1111/pbi.13121 pmid: 30963665 |
| [32] |
Li HY, Lv QY, Ma C, et al. Metabolite profiling and transcriptome analyses provide insights into the flavonoid biosynthesis in the developing seed of Tartary buckwheat(Fagopyrum tataricum)[J]. J Agric Food Chem, 2019, 67(40): 11262-11276.
doi: 10.1021/acs.jafc.9b03135 URL |
| [33] |
Zhang LJ, Li XX, Ma B, et al. The Tartary buckwheat genome provides insightsinto rutin biosynthesis and abiotic StressTolerance[J]. Mol Plant, 2017, 10(9): 1224-1237.
doi: 10.1016/j.molp.2017.08.013 URL |
| [34] | 吕丹, 黎瑞源, 郑冉, 等. 苦荞“翅米荞×野苦荞”重组自交系群体籽粒黄酮含量及籽粒性状的分析[J]. 贵州师范大学学报: 自然科学版, 2019, 37(6): 40-46. |
| Lv D, Li RY, Zheng R, et al. Analysis of flavonoids content in grains and grain traits on ‘Chimiqiao’ and ‘Yekuqiao’ recombinant inbred lines population of Tartary buckwheat[J]. J Guizhou Norm Univ Nat Sci, 2019, 37(6): 40-46. | |
| [35] |
Li CL, Bai YC, Li SJ, et al. Cloning, characterization, and activity analysis of a flavonol synthase gene FtFLS1 and its association with flavonoid content in Tartary buckwheat[J]. J Agric Food Chem, 2012, 60(20): 5161-5168.
doi: 10.1021/jf205192q URL |
| [36] |
Xu HT, Jiang ZQ, Lin ZM, et al. FtUGT79A15 is responsible for rutinosylation in flavonoid diglycoside biosynthesis in Fagopyrum tataricum[J]. Plant Physiol Biochem, 2022, 181: 33-41.
doi: 10.1016/j.plaphy.2022.04.004 URL |
| [37] |
Yin QG, Han XY, Han ZX, et al. Genome-wide analyses reveals a glucosyltransferase involved in rutin and emodin glucoside biosynthesis in Tartary buckwheat[J]. Food Chem, 2020, 318: 126478.
doi: 10.1016/j.foodchem.2020.126478 URL |
| [1] | 王佳蕊, 孙培媛, 柯瑾, 冉彬, 李洪有. 苦荞糖基转移酶基因FtUGT143的克隆及表达分析[J]. 生物技术通报, 2023, 39(8): 204-212. |
| [2] | 李博, 刘合霞, 陈宇玲, 周兴文, 朱宇林. 金花茶CnbHLH79转录因子的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 241-250. |
| [3] | 孙明慧, 吴琼, 刘丹丹, 焦小雨, 王文杰. 茶树CsTMFs的克隆与表达分析[J]. 生物技术通报, 2023, 39(7): 151-159. |
| [4] | 刘辉, 卢扬, 叶夕苗, 周帅, 李俊, 唐健波, 陈恩发. 外源硫诱导苦荞镉胁迫响应的比较转录组学分析[J]. 生物技术通报, 2023, 39(5): 177-191. |
| [5] | 张和臣, 袁欣, 高杰, 王校晨, 王慧娟, 李艳敏, 王利民, 符真珠, 李保印. 植物花瓣呈色机理及花色分子育种[J]. 生物技术通报, 2023, 39(5): 23-31. |
| [6] | 马芳芳, 刘冠闻, 庞冰, 蒋春美, 师俊玲. 强化细胞外排提高工程菌类黄酮产量的策略[J]. 生物技术通报, 2023, 39(5): 63-76. |
| [7] | 刘思佳, 王浩楠, 付宇辰, 闫文欣, 胡增辉, 冷平生. ‘西伯利亚’百合LiCMK基因克隆及功能分析[J]. 生物技术通报, 2023, 39(3): 196-205. |
| [8] | 郑敏敏, 柳洁, 赵清. 药用植物黄芩的生物学研究进展及展望[J]. 生物技术通报, 2023, 39(2): 10-23. |
| [9] | 杨旭妍, 赵爽, 马天意, 白玉, 王玉书. 三个甘蓝WRKY基因的克隆及其对非生物胁迫的表达[J]. 生物技术通报, 2023, 39(11): 261-269. |
| [10] | 侯瑞泽, 鲍悦, 陈启亮, 毛桂玲, 韦博霖, 侯雷平, 李梅兰. 普通白菜PRR5的克隆、表达及功能验证[J]. 生物技术通报, 2023, 39(10): 128-135. |
| [11] | 齐方婷, 黄河. 观赏植物花斑形成调控机制的研究进展[J]. 生物技术通报, 2023, 39(10): 17-28. |
| [12] | 杨敏, 龙雨青, 曾娟, 曾梅, 周新茹, 王玲, 付学森, 周日宝, 刘湘丹. 灰毡毛忍冬UGTPg17、UGTPg36基因克隆及功能研究[J]. 生物技术通报, 2023, 39(10): 256-267. |
| [13] | 关志秀, 汪燕, 梁成刚, 韦春玉, 黄娟, 陈庆富. 苦荞FtCBL基因的鉴定及对干旱与高钙胁迫的响应[J]. 生物技术通报, 2022, 38(8): 101-109. |
| [14] | 郭志浩, 金泽鑫, 刘琦, 高利. 小麦矮腥黑粉菌效应蛋白g11335的生物信息学分析、亚细胞定位及毒性验证[J]. 生物技术通报, 2022, 38(8): 110-117. |
| [15] | 李秀青, 胡子曜, 雷建峰, 代培红, 刘超, 邓嘉辉, 刘敏, 孙玲, 刘晓东, 李月. 棉花黄萎病抗性相关基因GhTIFY9的克隆与功能分析[J]. 生物技术通报, 2022, 38(8): 127-134. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||