








生物技术通报 ›› 2024, Vol. 40 ›› Issue (10): 305-314.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0384
韩雪1,2( ), 张阿娜3, 王海燕4, 辛凤姣1,2, 谷天一1,2(
), 张阿娜3, 王海燕4, 辛凤姣1,2, 谷天一1,2( ), 王钰璐1,2(
), 王钰璐1,2( )
)
收稿日期:2024-04-15
出版日期:2024-10-26
发布日期:2024-11-20
通讯作者:
王钰璐,女,博士,助理研究员,研究方向 :生物大分子结构与功能 ;E-mail: wnewyx@163.com;作者简介:韩雪,女,博士研究生,研究方向:食品酶学;E-mail: njhanxue1995@163.com张阿娜为本文共同第一作者
基金资助:
HAN Xue1,2( ), ZHANG A-na3, WANG Hai-yan4, XIN Feng-jiao1,2, GU Tian-yi1,2(
), ZHANG A-na3, WANG Hai-yan4, XIN Feng-jiao1,2, GU Tian-yi1,2( ), WANG Yu-lu1,2(
), WANG Yu-lu1,2( )
)
Received:2024-04-15
Published:2024-10-26
Online:2024-11-20
摘要:
【目的】瘤胃真菌Neocallimastix patriciarum GH11家族木聚糖酶CDBFV在饲料、食品等领域具有良好应用前景,提高其热稳定性对其生产应用十分重要。【方法】使用分子动力学模拟、机器学习等策略设计潜在CDBFV热稳定性突变体,在大肠杆菌和毕赤酵母中进行异源表达纯化,测定最适反应条件、比酶活和85℃孵育3 min后相对剩余活力,通过结构分析明确热稳定性提高机制。【结果】位于CDBFV N端基序36GNNS39具有较高柔性,对其改造设计的单突变体N37P和N38V在85℃孵育3 min后,相对活力分别降低至70.3%和55.1%,较野生型(48.7%)提升了21.6%和6.5%;进而,在相对活力提升显著的N37P基础上叠加已报道优势突变体N88G,构建双突变体N37P/N88G,其相对活力达到了73.4%,较野生型提高了24.7%;此外,将N37P/N88G在毕赤酵母中进行了分泌表达,在85℃处理3 min后,相对活力达到了88.8%;结构分析表明,N37P突变的引入使得CDBFV形成了新的氢键相互作用,降低活性位点附近柔性,并干预了糖基化形成,进而提高了热稳定性。【结论】成功得到了高耐热双突变体N37P/N88G,为提高GH11家族木聚糖酶的热稳定性改造提供了新的思路和方法,有望推动CDBFV在饲料工业等高温环境下的广泛应用。
韩雪, 张阿娜, 王海燕, 辛凤姣, 谷天一, 王钰璐. 基于计算设计的GH11家族木聚糖酶CDBFV的热稳定性改造及潜在机制研究[J]. 生物技术通报, 2024, 40(10): 305-314.
HAN Xue, ZHANG A-na, WANG Hai-yan, XIN Feng-jiao, GU Tian-yi, WANG Yu-lu. Computer-aided Thermostability Engineering and Underlying Mechanism Investigation of the GH11 Family Xylanase CDBFV[J]. Biotechnology Bulletin, 2024, 40(10): 305-314.
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') |
|---|---|
| N37P-F | CGGATAGCGGCCCGAATAGCGCGACCTTTTATA-G |
| N37P-R | CGCGCTATTCGGGCCGCTATCCGCCCACAGTTCATAG |
| N38V-F | CGGATAGCGGCAATGTTAGCGCGACCTTTTATAGCGATGGC |
| N38V-R | GGTCGCGCTGTTATTGCCGCTATCCGCCCACAGTTCATAG |
| N88G-F | CTGGTGAAACAGGGTAGCAGCAATGTGGGCTATAGCTATG |
| N88G-R | GCCCACATTGCTGCTACCCTGTTTCACCAGTTTAAAATCCGC |
表1 定点突变所用引物
Table 1 Primers used by site-directed mutagenesis
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') |
|---|---|
| N37P-F | CGGATAGCGGCCCGAATAGCGCGACCTTTTATA-G |
| N37P-R | CGCGCTATTCGGGCCGCTATCCGCCCACAGTTCATAG |
| N38V-F | CGGATAGCGGCAATGTTAGCGCGACCTTTTATAGCGATGGC |
| N38V-R | GGTCGCGCTGTTATTGCCGCTATCCGCCCACAGTTCATAG |
| N88G-F | CTGGTGAAACAGGGTAGCAGCAATGTGGGCTATAGCTATG |
| N88G-R | GCCCACATTGCTGCTACCCTGTTTCACCAGTTTAAAATCCGC |

图1 序列分析及突变位点的筛选 A:野生型CDBFV在298 K和360 K下不同残基的RMSF值分析;B:野生型CDBFV与同源木聚糖酶的N端序列比对,XynSW1、Xyn11B、Xyn11NX和PVX分别来自链霉菌、黑曲霉、新疆涅斯捷连科氏菌和拟青霉属,绿色框为基序36GNNS39;C:柔性区域在结构中的位置,黄色表示RMSF值较高的柔性区域151SIDGD155和36GNNS39
Fig. 1 Sequence analysis and screening of mutation sites A: Analysis of RMSF values of wild-type CDBFV at 298 K and 360 K. B: N-terminal sequence alignment of wild-type CDBFV with homologous xylanases, XynSW1, Xyn11B, Xyn11NX and PVX from Streptomyces sp., Aspergillus niger, Nesterenkonia xinjiangensis and Paecilonyces variotii, respectively. Green box refers to motif 36GNNS39. C: The flexible region 151SIDGD155和36GNNS39 with high RMSF value is highlighted in yellow in the structure
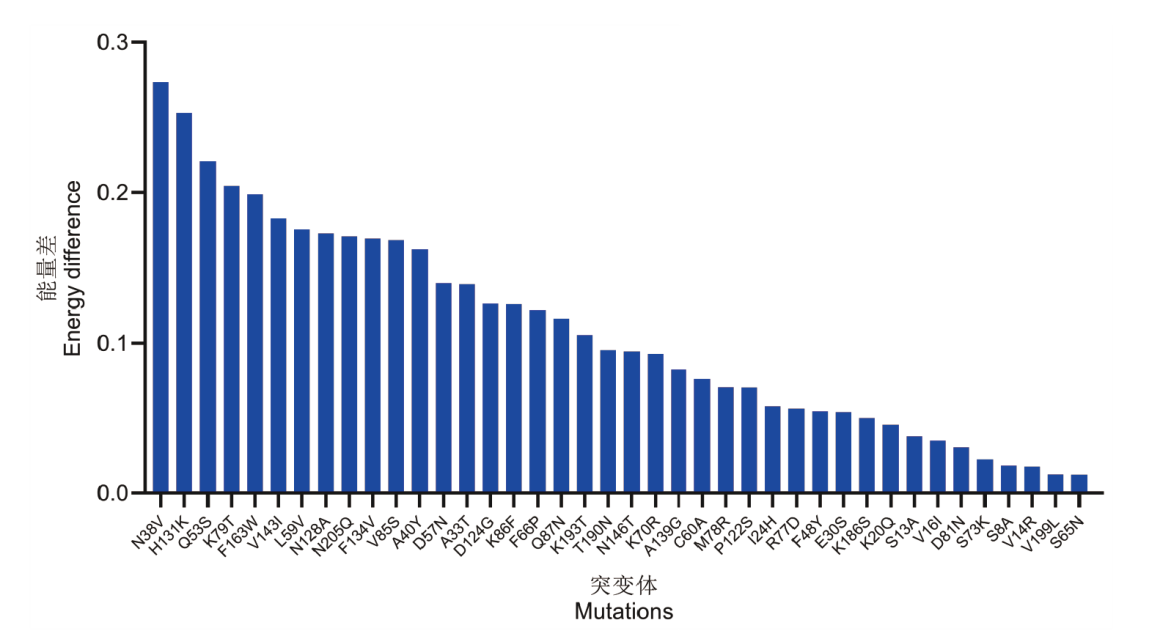
图2 机器学习模型预测能量排名 机器学习模型预测后高温条件下最保守氨基酸分值减去野生型氨基酸分值的大小排序
Fig. 2 Machine learning-based energy ranking prediction The figure illustrates the ranking of the most conserved amino acid scores at high temperature minus the wild-type amino acid scores predicted by a machine learning model
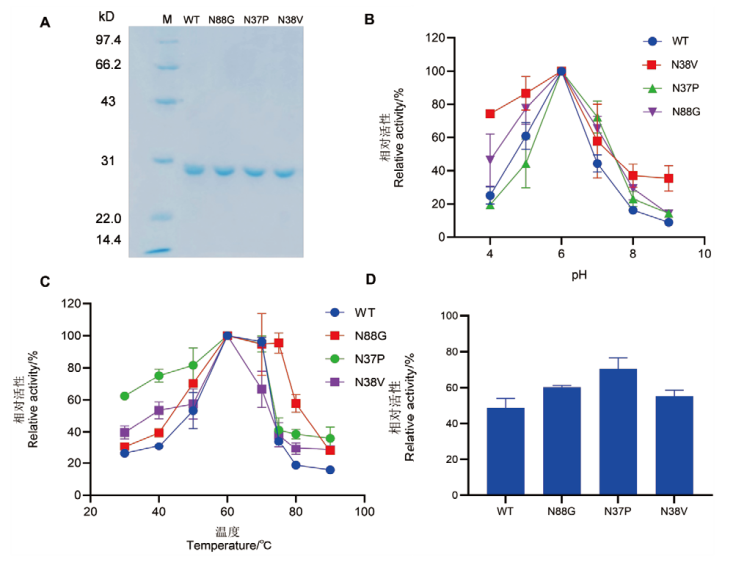
图3 野生型及单突变体SDS-PAGE及酶学性质 A:野生型CDBFV及单突变体的SDS-PAGE分析,M:标准分子量蛋白Marker;B:野生型及单突变体的最适pH;C:野生型及单突变体的最适温度;D:野生型及单突变体在85℃处理3 min较处理前相对酶活力
Fig. 3 SDS-PAGE analysis and enzymatic properties of wild-type and single mutants A: SDS-PAGE analysis of wild type CDBFV and single mutant; M: standard molecular weight protein marker. B: The optimal pH of wild type and single mutants. C: The optimal temperature of wild type and single mutants. D: The relative enzyme activity of wild type and single mutants after treatment at 85℃ for 3 min compared to their pre-treatment states
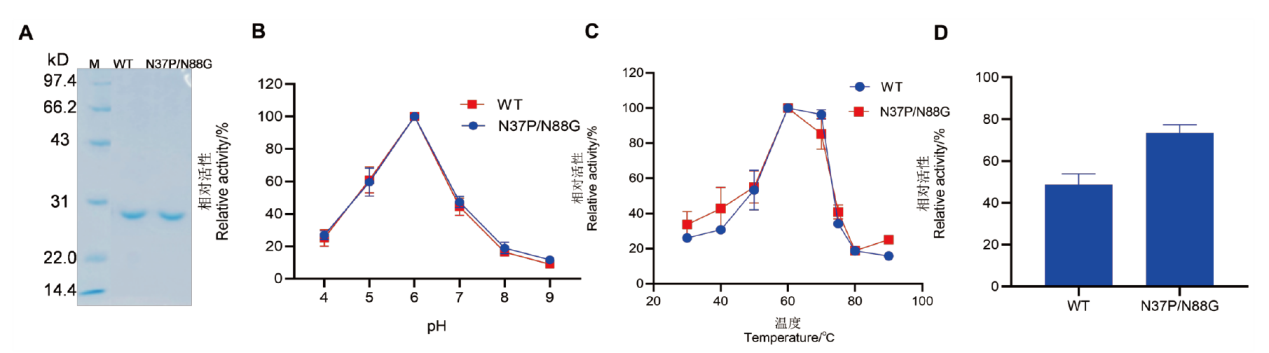
图4 野生型及双突变体SDS-PAGE及酶学性质 A:野生型CDBFV及双突变体N37P/N88G的SDS-PAGE分析,M:标准分子量蛋白Marker;B:野生型及双突变体的最适pH;C:野生型及双突变体的最适温度;D:野生型及双突变体在85℃处理3 min较处理前相对酶活力
Fig. 4 SDS-PAGE analysis and enzymatic properties of wild-type and the double mutant A: SDS-PAGE analysis of wild type CDBFV and N37P/N88G double mutant; M: standard molecular weight protein marker. B: The optimal pH of wild type and the double mutant. C: The optimal temperature of wild type and the double mutant. D: The relative enzyme activity of wild type and the double mutant after treatment at 85℃ for 3 min compared to their pre-treatment states
| 酶Enzyme | 最适pH Optimal pH | 最适温度Optimal temperature/℃ | 绝对酶活Specific activity/(U·mg-1) | 相对酶活a Relative activity/% |
|---|---|---|---|---|
| WT(E. coli) | 6 | 60 | 5 216.2 | 48.7 |
| N37P(E. coli) | 6 | 60 | 1 108 | 70.3 |
| N38V(E. coli) | 6 | 60 | 2 130.45 | 55.1 |
| N37P/N88G(E. coli) | 6 | 60 | 807.2 | 73.4 |
| WT(P. pastoris) | 5 | 70 | 1 050 | 76.6 |
| N37P/N88G(P. pastoris) | 5 | 70 | 900 | 88.8 |
表2 CDBFV野生型和突变体的最适条件及酶活测定
Table 2 Optimal condition and enzymatic activity determination of CDBFV wild-type and mutants
| 酶Enzyme | 最适pH Optimal pH | 最适温度Optimal temperature/℃ | 绝对酶活Specific activity/(U·mg-1) | 相对酶活a Relative activity/% |
|---|---|---|---|---|
| WT(E. coli) | 6 | 60 | 5 216.2 | 48.7 |
| N37P(E. coli) | 6 | 60 | 1 108 | 70.3 |
| N38V(E. coli) | 6 | 60 | 2 130.45 | 55.1 |
| N37P/N88G(E. coli) | 6 | 60 | 807.2 | 73.4 |
| WT(P. pastoris) | 5 | 70 | 1 050 | 76.6 |
| N37P/N88G(P. pastoris) | 5 | 70 | 900 | 88.8 |
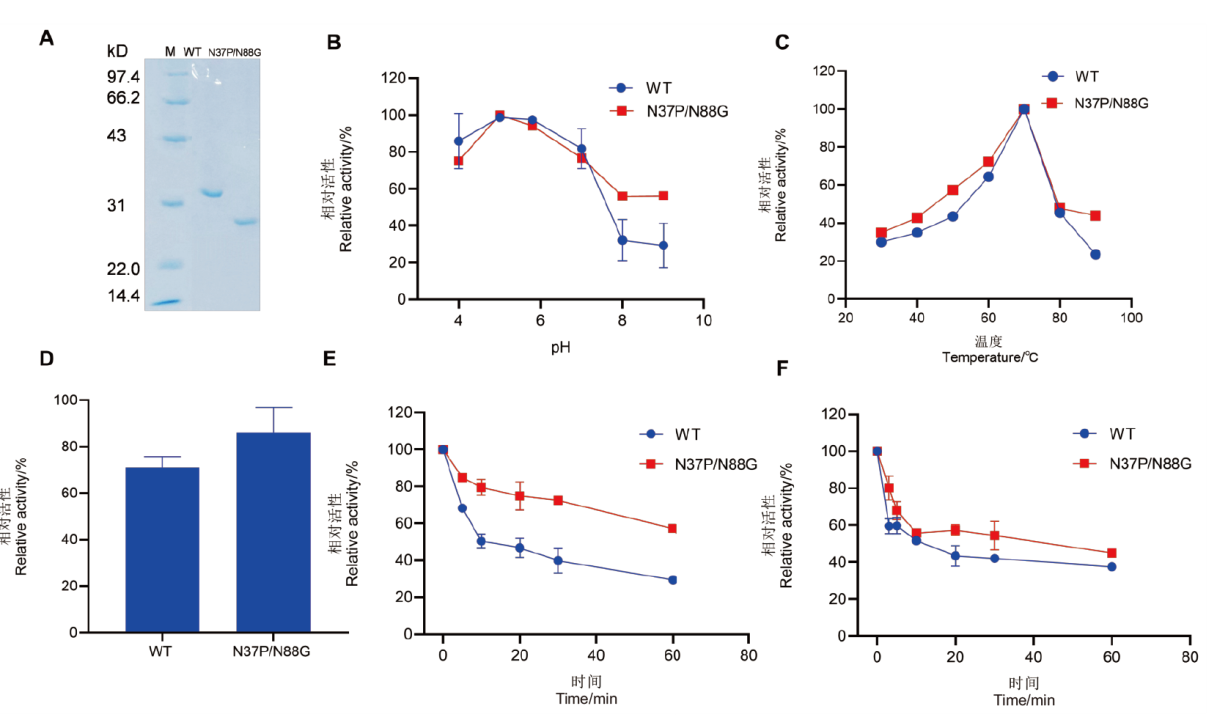
图5 毕赤酵母表达的野生型及双突变体SDS-PAGE及酶学性质 A:野生型及双突变体的SDS-PAGE分析,M:标准分子量蛋白Marker;B:野生型及双突变体的最适pH;C:野生型及双突变体的最适温度;D:野生型及双突变体在85℃处理3 min较处理前相对酶活力;E:野生型及双突变体在70℃处理0-60 min的温度稳定性;F:野生型及双突变体在80℃处理0-60 min的温度稳定性
Fig. 5 SDS-PAGE analysis and enzymatic properties of wild-type and the double mutant expressed in Pichia pastoris A: SDS-PAGE analysis of wild type and double mutant; M: standard molecular weight protein marker. B: Optimal pH of wild type and the double mutant. C: Optimal temperature of wild type and the double mutant. D: The relative enzyme activity of wild type and the double mutant after treatment at 85℃ for 3 min compared to their pre-treatment states. E: Temperature stability of wild type and the double mutant after treatment at 70℃ for 0-60 min. F: Temperature stability of wild type and the double mutant after treatment at 80℃ for 0-60 min

图6 野生型和双突变体N88G/N37P的结构分析 A:野生型和突变体N88G的表面电荷分析;蓝色为正电荷,红色为负电荷,白色不带电荷;B:野生型和突变体N37P的形成的相互作用分析;蓝色线表示氨基酸之间的氢键相互作用
Fig. 6 Structural analysis of the wild-type and double mutant N88G/N37P A: Analysis of the surface potential of the wild-type and mutant N88G mutant; blue, red and white colors correspond to positive, negative and uncharged regions, respectively. B: Amino acid interaction analysis of the wild-type and mutant N37P; the blue line indicates the hydrogen bond interactions
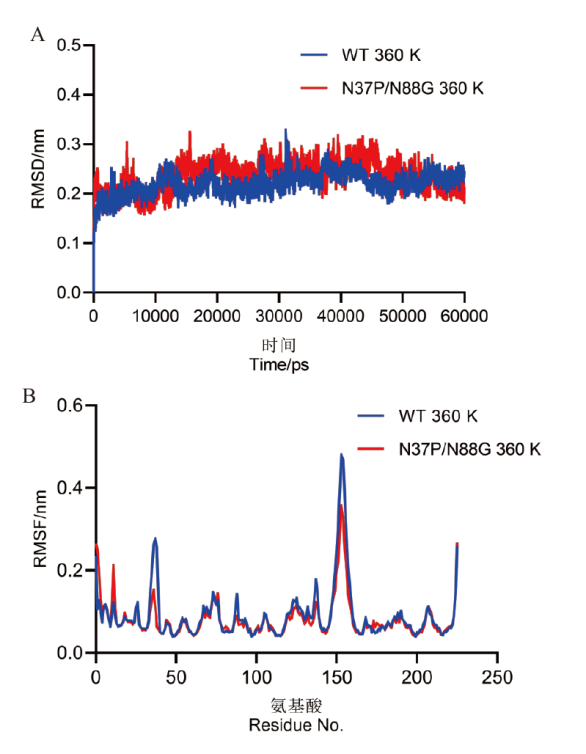
图7 野生型及双突变体360 K的分子动力学模拟 A:野生型及双突变体在360 K下的模拟50 ns RMSD值分析;B:野生型及双突变体在360 K下不同残基的RMSF值分析
Fig. 7 Molecular dynamic simulation of the wild-type and double mutant at 360 K A: Analysis of the RMSD values for the wild-type and double mutant during a 50 ns simulation at 360 K. B: Analysis of the RMSF values for different residues in the wild-type and double mutant at 360 K
| [1] |
Collins T, Gerday C, Feller G. Xylanases, xylanase families and extremophilic xylanases[J]. FEMS Microbiol Rev, 2005, 29(1): 3-23.
doi: 10.1016/j.femsre.2004.06.005 pmid: 15652973 |
| [2] |
Subramaniyan S, Prema P. Biotechnology of microbial xylanases: enzymology, molecular biology, and application[J]. Crit Rev Biotechnol, 2002, 22(1): 33-64.
doi: 10.1080/07388550290789450 pmid: 11958335 |
| [3] | Mamo G. Alkaline active hemicellulases[J]. Adv Biochem Eng Biotechnol, 2020, 172: 245-291. |
| [4] |
Polizeli MLTM, Rizzatti ACS, Monti R, et al. Xylanases from fungi: properties and industrial applications[J]. Appl Microbiol Biotechnol, 2005, 67(5): 577-591.
doi: 10.1007/s00253-005-1904-7 pmid: 15944805 |
| [5] | Fernandes de Souza H, Aguiar Borges L, Dédalo Di Próspero Gonçalves V, et al. Recent advances in the application of xylanases in the food industry and production by Actinobacteria: a review[J]. Food Res Int, 2022, 162(Pt B): 112103. |
| [6] | Murthy PS, Naidu MM. Production and application of xylanase from Penicillium sp. utilizing coffee by-products[J]. Food Bioprocess Technol, 2012, 5(2): 657-664. |
| [7] | Motta FL, Andrade CCP, Sant MHA. A review of xylanase production by the fermentation of xylan: classification, characterization and applications[M]//Sustainable Degradation of Lignocellulosic Biomass - Techniques, Applications and Commercialization.: InTech, 2013. |
| [8] |
Woyengo TA, Sands JS, Guenter W, et al. Nutrient digestibility and performance responses of growing pigs fed phytase- and xylanase-supplemented wheat-based diets[J]. J Anim Sci, 2008, 86(4): 848-857.
doi: 10.2527/jas.2007-0018 pmid: 18203976 |
| [9] | Khandeparkar R, Bhosle NB. Application of thermoalkalophilic xylanase from Arthrobacter sp. MTCC 5214 in biobleaching of kraft pulp[J]. Bioresour Technol, 2007, 98(4): 897-903. |
| [10] | Liu SY, Zhuang XH, Zhang XL, et al. Enzymatic modification of rice bran polysaccharides by enzymes from Grifola frondosa: natural killer cell cytotoxicity and antioxidant activity[J]. J Food Sci, 2018, 83(7): 1948-1955. |
| [11] | Zhang Y, Guan FF, Xu GS, et al. A novel thermophilic chitinase directly mined from the marine metagenome using the deep learning tool Preoptem[J]. Bioresour Bioprocess, 2022, 9(1): 54. |
| [12] | Newberry RW, Raines RT. The n→π* interaction[J]. Acc Chem Res, 2017, 50(8): 1838-1846. |
| [13] | Han NY, Ma Y, Mu YL, et al. Enhancing thermal tolerance of a fungal GH11 xylanase guided by B-factor analysis and multiple sequence alignment[J]. Enzyme Microb Technol, 2019, 131: 109422. |
| [14] | Bajaj P, Mahajan R. Cellulase and xylanase synergism in industrial biotechnology[J]. Appl Microbiol Biotechnol, 2019, 103(21/22): 8711-8724. |
| [15] |
Nawab A, Ibtisham F, Li GH, et al. Heat stress in poultry production: mitigation strategies to overcome the future challenges facing the global poultry industry[J]. J Therm Biol, 2018, 78: 131-139.
doi: S0306-4565(18)30130-X pmid: 30509629 |
| [16] |
刘伟, 于凤民, 马千鹏, 等. 木聚糖酶在青贮及反刍动物日粮中的应用[J]. 草地学报, 2024, 32(1): 331-339.
doi: 10.11733/j.issn.1007-0435.2024.01.034 |
|
Liu W, Yu FM, Ma QP, et al. Application of xylanase in silage and ruminant diet[J]. Acta Agrestia sinica, 2024, 32(1): 331-339.
doi: 10.11733/j.issn.1007-0435.2024.01.034 |
|
| [17] | Alponti JS, Fonseca Maldonado R, Ward RJ. Thermostabilization of Bacillus subtilis GH11 xylanase by surface charge engineering[J]. Int J Biol Macromol, 2016, 87: 522-528. |
| [18] | Mendonça M, Barroca M, Collins T. Endo-1, 4-β-xylanase-containing glycoside hydrolase families: characteristics, singularities and similarities[J]. Biotechnol Adv, 2023, 65: 108148. |
| [19] | Chadha BS, Kaur B, Basotra N, et al. Thermostable xylanases from thermophilic fungi and bacteria: current perspective[J]. Bioresour Technol, 2019, 277: 195-203. |
| [20] | Zhang S, Zhang K, Chen XZ, et al. Five mutations in N-terminus confer thermostability on mesophilic xylanase[J]. Biochem Biophys Res Commun, 2010, 395(2): 200-206. |
| [21] | Wu H, Chen QM, Zhang WL, et al. Overview of strategies for developing high thermostability industrial enzymes: discovery, mechanism, modification and challenges[J]. Crit Rev Food Sci Nutr, 2023, 63(14): 2057-2073. |
| [22] | Li YY, Li C, Huang H, et al. Significantly enhanced thermostability of Aspergillus niger xylanase by modifying its highly flexible regions[J]. J Agric Food Chem, 2022, 70(15): 4620-4630. |
| [23] | You C, Huang Q, Xue HP, et al. Potential hydrophobic interaction between two cysteines in interior hydrophobic region improves thermostability of a family 11 xylanase from Neocallimastix patriciarum[J]. Biotechnol Bioeng, 2010, 105(5): 861-870. |
| [24] | Wu QH, Zhang CN, Dong WQ, et al. Simultaneously enhanced thermostability and catalytic activity of xylanase from Streptomyces rameus L2001 by rigidifying flexible regions in loop regions of the N-terminus[J]. J Agric Food Chem, 2023, 71(34): 12785-12796. |
| [25] | Bhat SK, Purushothaman K, Kini KR, et al. Design of mutants of GH11 xylanase from Bacillus pumilus for enhanced stability by amino acid substitutions in the N-terminal region: an in silico analysis[J]. J Biomol Struct Dyn, 2022, 40(17): 7666-7679. |
| [26] |
Foroozandeh Shahraki M, Farhadyar K, Kavousi K, et al. A generalized machine-learning aided method for targeted identification of industrial enzymes from metagenome: A xylanase temperature dependence case study[J]. Biotechnol Bioeng, 2021, 118(2): 759-769.
doi: 10.1002/bit.27608 pmid: 33095441 |
| [27] |
Eijsink VGH, Bjørk A, Gåseidnes S, et al. Rational engineering of enzyme stability[J]. J Biotechnol, 2004, 113(1-3): 105-120.
doi: 10.1016/j.jbiotec.2004.03.026 pmid: 15380651 |
| [28] |
Zhou XX, Wang YB, Pan YJ, et al. Differences in amino acids composition and coupling patterns between mesophilic and thermophilic proteins[J]. Amino Acids, 2008, 34(1): 25-33.
pmid: 17710363 |
| [29] |
Karbalaei M, Rezaee SA, Farsiani H. Pichia pastoris: a highly successful expression system for optimal synthesis of heterologous proteins[J]. J Cell Physiol, 2020, 235(9): 5867-5881.
doi: 10.1002/jcp.29583 pmid: 32057111 |
| [30] | 朱泰承, 李寅. 毕赤酵母表达系统发展概况及趋势[J]. 生物工程学报, 2015, 31(6): 929-938. |
| Zhu TC, Li Y. Recent development of Pichia pastoris system: current status and future perspective[J]. Chin J Biotechnol, 2015, 31(6): 929-938. | |
| [31] |
Vogt G, Woell S, Argos P. Protein thermal stability, hydrogen bonds, and ion pairs[J]. J Mol Biol, 1997, 269(4): 631-643.
doi: 10.1006/jmbi.1997.1042 pmid: 9217266 |
| [1] | 张阿娜, 韩雪, 谷天一, 辛凤姣, 王钰璐. 利用新型红酵母苯丙氨酸解氨酶制备低苯丙氨酸酪蛋白[J]. 生物技术通报, 2024, 40(8): 309-319. |
| [2] | 乔烨, 张楠, 杨建花, 张翠英, 朱蕾蕾. 糖磷酸酶的挖掘及其酶学性质研究[J]. 生物技术通报, 2024, 40(7): 299-306. |
| [3] | 蔡逸安, 张轶群, 杨子璇, 刘业学, 刘文龙, 路福平, 李玉. 分子伴侣增强蛋白酶K在毕赤酵母中的表达及对羊毛鳞片层的作用分析[J]. 生物技术通报, 2024, 40(7): 307-313. |
| [4] | 蒋文萍, 冉秋萍, 刘家书, 张慧敏, 张迪, 江正兵, 李华南. 碳水化合物结合域对木聚糖酶酶学性质的影响[J]. 生物技术通报, 2024, 40(5): 269-279. |
| [5] | 茹扎·也里扎提, 杨宇. 毕赤酵母中外源蛋白表达量的提升策略[J]. 生物技术通报, 2024, 40(3): 118-134. |
| [6] | 夏光丽, 曹娜, 孙慧慧, 赵玲, 曹荣. 半乳糖氧化酶的生物学改造研究进展[J]. 生物技术通报, 2024, 40(1): 176-185. |
| [7] | 赵思佳, 王晓璐, 孙纪录, 田健, 张杰. 代谢工程改造毕赤酵母生产赤藓糖醇[J]. 生物技术通报, 2023, 39(8): 137-147. |
| [8] | 董聪, 高庆华, 王玥, 罗同阳, 王庆庆. 基于联合策略提高FAD依赖的葡萄糖脱氢酶的酵母表达[J]. 生物技术通报, 2023, 39(6): 316-324. |
| [9] | 曲戈, 孙周通. 催化混杂性驱动的酶功能重塑[J]. 生物技术通报, 2023, 39(4): 1-9. |
| [10] | 赵昕, 杜玉瑶, 殷子扬, 毛淑红. 胆固醇7α-羟化酶在毕赤酵母中的异源表达[J]. 生物技术通报, 2023, 39(10): 304-310. |
| [11] | 王玥, 高庆华, 董聪, 罗同阳, 王庆庆. 密码子优化的吡喃糖氧化酶基因在毕赤酵母中的表达[J]. 生物技术通报, 2022, 38(4): 269-277. |
| [12] | 李志豪, 张鸽, 貊志杰, 邓帅军, 李佳轶, 张海波, 刘晓晖, 刘好宝. 一株产木聚糖酶的蜡样芽孢杆菌对雪茄烟叶成分及发酵产物的影响[J]. 生物技术通报, 2022, 38(2): 105-112. |
| [13] | 张晨, 张佟佟, 刘海萍. 高活性和高热稳定性乙烯合成酶的筛选和鉴定[J]. 生物技术通报, 2022, 38(11): 269-276. |
| [14] | 杨威, 伍茜, 程建国, 罗燕, 王印, 杨泽晓, 姚学萍. 林麝干扰素α基因克隆、表达及转录调控分析[J]. 生物技术通报, 2022, 38(1): 194-204. |
| [15] | 廖兆民, 蔡俊, 林建国, 杜馨, 王常高. 黑曲霉葡萄糖氧化酶基因在毕赤酵母中的表达及产酶条件的优化[J]. 生物技术通报, 2021, 37(6): 97-107. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||