








生物技术通报 ›› 2025, Vol. 41 ›› Issue (7): 95-105.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0109
牛景萍1( ), 赵婧2, 郭茜2, 王书宏2, 赵晋忠3, 杜维俊2, 殷丛丛3, 岳爱琴2(
), 赵婧2, 郭茜2, 王书宏2, 赵晋忠3, 杜维俊2, 殷丛丛3, 岳爱琴2( )
)
收稿日期:2025-01-26
出版日期:2025-07-26
发布日期:2025-07-22
通讯作者:
岳爱琴,女,博士,教授,研究方向 :大豆遗传与种质创新;E-mail: yueaiqinnd@126.com作者简介:牛景萍,女,博士,讲师,研究方向 :大豆抗病;E-mail: niujingping@sxau.edu.cn
基金资助:
NIU Jing-ping1( ), ZHAO Jing2, GUO Qian2, WANG Shu-hong2, ZHAO Jin-zhong3, DU Wei-jun2, YIN Cong-cong3, YUE Ai-qin2(
), ZHAO Jing2, GUO Qian2, WANG Shu-hong2, ZHAO Jin-zhong3, DU Wei-jun2, YIN Cong-cong3, YUE Ai-qin2( )
)
Received:2025-01-26
Published:2025-07-26
Online:2025-07-22
摘要:
目的 挖掘大豆抗大豆花叶病毒(soybean mosaic virus, SMV)病相关的核心转录因子(transcription factors, TFs),为深入解析大豆抗病分子机制和创制抗病品种提供理论依据。 方法 以大豆抗病材料X149和感病材料X97受大豆花叶病毒株系SC15诱导后的转录组数据为基础,利用PlantTFDB v 5.0数据库从转录组中预测全基因组转录因子基因;基于差异表达转录因子基因,通过加权基因共表达网络分析(weighted gene co-expression network analysis, WGCNA)获得抗病转录因子相关模块和抗病核心转录因子;采用String 12.0预测转录因子互作蛋白;利用RT-qPCR分析转录因子受激素(ETH、SA、MeJA和ABA)诱导表达情况。 结果 全基因组含有转录因子的基因有3 170个,其中1 727个属于差异表达基因;WGCNA分析表明1 727个基因被划分为6个转录因子基因共表达模块,其中brown模块和turquoise模块与X149抗性显著相关,2个模块中转录因子基因间连通度最高的核心转录因子为turquoise模块中NAC转录因子GmNAC030基因,turquoise模块中与GmNAC030具有相似表达的NAC转录因子基因有GmNAC043、GmNAC085、GmNAC092和GmNAC101;互作蛋白预测表明,GmNAC030、GmNAC043和GmNAC092互作蛋白均为转录因子,且仅有互作蛋白Glyma.02g131700(bZIP1)、Glyma.16g164800(AP2-EREBP)和Glyma.08G118200(WRKY48)位于模块中。RT-qPCR分析表明GmNAC030主要受MeJA诱导,另外4个NAC转录因子基因主要受MeJA和ETH诱导。 结论 5个NAC转录因子基因GmNAC030、GmNAC043、GmNAC085、GmNAC092和GmNAC101的表达与大豆抗SMV相关且受外源激素MeJA和ETH的诱导。
牛景萍, 赵婧, 郭茜, 王书宏, 赵晋忠, 杜维俊, 殷丛丛, 岳爱琴. 基于WGCNA鉴定大豆抗大豆花叶病毒NAC转录因子及其诱导表达分析[J]. 生物技术通报, 2025, 41(7): 95-105.
NIU Jing-ping, ZHAO Jing, GUO Qian, WANG Shu-hong, ZHAO Jin-zhong, DU Wei-jun, YIN Cong-cong, YUE Ai-qin. Identification and Induced Expression Analysis of Transcription Factors NAC in Soybean Resistance to Soybean Mosaic Virus Based on WGCNA[J]. Biotechnology Bulletin, 2025, 41(7): 95-105.
基因名称 Gene name | 基因ID Gene ID | 上游引物 Forward primer (5′-3′) | 下游引物 Reverse primer (5′-3′) |
|---|---|---|---|
| GmNAC030 | Glyma.05G195000 | GGTTCAGTTCCAGCACGAGA | CGTGTCCATGTACAATTGGTCG |
| GmNAC043 | Glyma.06G248900 | TCTTCCCGCAAACACAACCT | TGACCCATTGCTGCATTCCA |
| GmNAC085 | Glyma.12G149100 | CGACATGCTGGAATCGTTGC | GTCCTGCTGCTGCATTGTTC |
| GmNAC092 | Glyma.12G221500 | ACAACTCGACGACGTTCTGG | CCCCCTTCACCCAAGTTCTG |
| GmNAC101 | Glyma.13G279900 | AACACGCTGCAACAACAACA | ATTCCCCGAAGCGAAATCGG |
| tublin | GGAGTTCACAGAGGCAGAG | CACTTACGCATCACATAGC |
表1 RT-qPCR引物信息
Table 1 Information of primers for RT-qPCR
基因名称 Gene name | 基因ID Gene ID | 上游引物 Forward primer (5′-3′) | 下游引物 Reverse primer (5′-3′) |
|---|---|---|---|
| GmNAC030 | Glyma.05G195000 | GGTTCAGTTCCAGCACGAGA | CGTGTCCATGTACAATTGGTCG |
| GmNAC043 | Glyma.06G248900 | TCTTCCCGCAAACACAACCT | TGACCCATTGCTGCATTCCA |
| GmNAC085 | Glyma.12G149100 | CGACATGCTGGAATCGTTGC | GTCCTGCTGCTGCATTGTTC |
| GmNAC092 | Glyma.12G221500 | ACAACTCGACGACGTTCTGG | CCCCCTTCACCCAAGTTCTG |
| GmNAC101 | Glyma.13G279900 | AACACGCTGCAACAACAACA | ATTCCCCGAAGCGAAATCGG |
| tublin | GGAGTTCACAGAGGCAGAG | CACTTACGCATCACATAGC |
转录因子家族 TF family | 基因数 Gene number | 转录因子家族 TF family | 基因数 Gene number |
|---|---|---|---|
| MYB | 335 | MIKC | 84 |
| ERF | 305 | Dof | 79 |
| bHLH | 295 | C3H | 69 |
| MYB_related | 266 | GATA | 60 |
| HB-other | 204 | ARF | 55 |
| WRKY | 176 | TCP | 54 |
| NAC | 171 | C2H2 | 53 |
| M_type | 144 | HSF | 52 |
| bZIP | 144 | FAR1 | 49 |
| B3 | 131 | ZF-HD | 47 |
| GRAS | 111 | SBP | 45 |
| LBD | 91 | AP2 | 44 |
| HD-ZIP | 41 | CPP | 12 |
| DBB | 34 | EIL | 11 |
| Nin-like | 28 | BBR-BPC | 10 |
| CO-like | 22 | GeBP | 9 |
| GRF | 22 | LSD | 8 |
| NF-YA | 21 | Whirly | 7 |
| SRS | 21 | HB-PHD | 6 |
| TALE | 18 | NF-X1 | 5 |
| YABBY | 17 | RAV | 4 |
| BES1 | 15 | S1Fa-like | 4 |
| CAMTA | 15 | LFY | 2 |
| E2F/DP | 14 | NZZ/SPL | 1 |
表2 转录因子统计
Table 2 Statistics of transcription factors (TF)
转录因子家族 TF family | 基因数 Gene number | 转录因子家族 TF family | 基因数 Gene number |
|---|---|---|---|
| MYB | 335 | MIKC | 84 |
| ERF | 305 | Dof | 79 |
| bHLH | 295 | C3H | 69 |
| MYB_related | 266 | GATA | 60 |
| HB-other | 204 | ARF | 55 |
| WRKY | 176 | TCP | 54 |
| NAC | 171 | C2H2 | 53 |
| M_type | 144 | HSF | 52 |
| bZIP | 144 | FAR1 | 49 |
| B3 | 131 | ZF-HD | 47 |
| GRAS | 111 | SBP | 45 |
| LBD | 91 | AP2 | 44 |
| HD-ZIP | 41 | CPP | 12 |
| DBB | 34 | EIL | 11 |
| Nin-like | 28 | BBR-BPC | 10 |
| CO-like | 22 | GeBP | 9 |
| GRF | 22 | LSD | 8 |
| NF-YA | 21 | Whirly | 7 |
| SRS | 21 | HB-PHD | 6 |
| TALE | 18 | NF-X1 | 5 |
| YABBY | 17 | RAV | 4 |
| BES1 | 15 | S1Fa-like | 4 |
| CAMTA | 15 | LFY | 2 |
| E2F/DP | 14 | NZZ/SPL | 1 |

图2 样本聚类图A:36个样本聚类图,3个红色的为离群样本;B:剔除离群样本后的33个样本聚类图
Fig. 2 Clustering diagram of samplesA: Clustering diagram of all 36 samples. The three outlier samples are written in red fonts. B: Clustering diagram of 33 samples after removing the three outlier samples

图3 加权基因共表达网络分析软阈值的确定和模块鉴定A:软阈值(β值)的确定;B:基因模块分类,树枝代表基因,不同颜色代表不同模块;C:模块与抗性表型相关热图,格子上方数字代表相关系数,下方数值是P值
Fig. 3 Determination of soft threshold power (β value) via WGCNA and identification of modulesA: Determination of soft threshold power (β value). B: Gene module classification, dendrogram indicate genes and different colors indicate different modules. C: Heat map of correlations between resistance trait and modules. The correlation coefficients are shown above each cell, and the P values are shown below

图4 抗病相关模块连通性前30个转录因子基因的可视化网络分析
Fig. 4 Visual network analysis of top 30 transcription factor genes in the disease resistance-related module connectivity

图5 NAC表达量热图和RT-qPCR验证A:5个NAC在X149和X97不同时间点的表达量热图;B:GmNAC030在X149中的RT-qPCR验证;*:P<0.05,**:P<0.01,下同
Fig. 5 Expression profiles of NAC and validation of NAC by RT-qPCRA: Expression profiles of five NAC at different time point X149 and X97. B: Validation of GmNAC030 in X149 by RT-qPCR; *: P<0.05, **: P<0.01,The same below
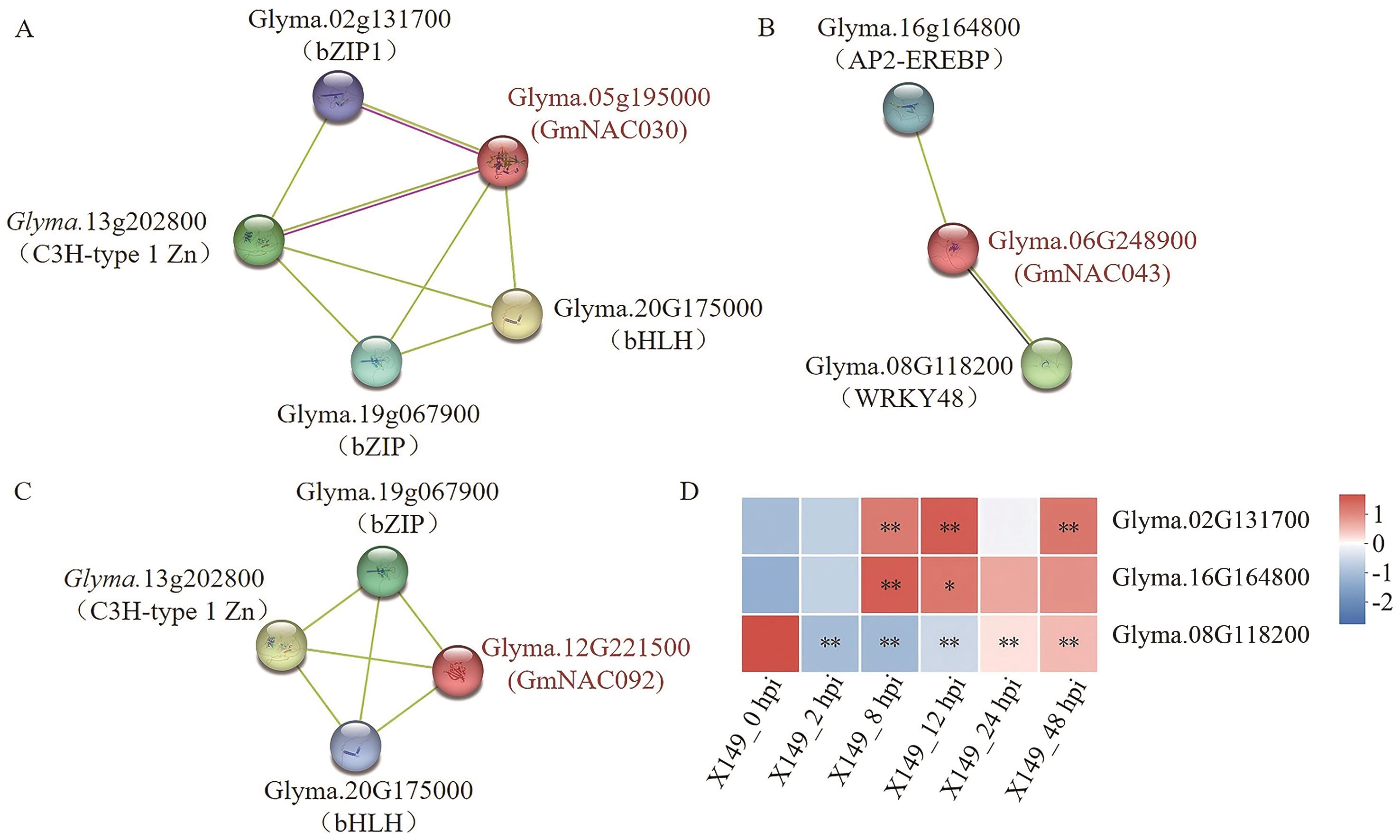
图6 NAC蛋白互作分析及互作蛋白在转录组中表达量热图A: Glyma.05g195000互作蛋白;B:Glyma.06G248900互作蛋白;C:Glyma.12G221500互作蛋白;D:存在于模块中的互作蛋白表达量热图
Fig. 6 Analysis of NAC protein interaction and expression profiles of interacting proteins in transcriptomeA: Interacting protein Glyma.05g195000. B: Interacting protein Glyma.06G248900. C: Interacting protein Glyma.12G221500. D: Expression profiles of interacting protein in the modules
| [1] | 王大刚, 李凯, 智海剑. 大豆抗大豆花叶病毒病基因研究进展 [J]. 中国农业科学, 2018, 51(16): 3040-3059. |
| Wang DG, Li K, Zhi HJ. Progresses of resistance on soybean mosaic virus in soybean [J]. Sci Agric Sin, 2018, 51(16): 3040-3059. | |
| [2] | 高乐, 李志强, 李凯, 等. 大豆花叶病毒病转基因抗性研究进展 [J]. 中国油料作物学报, 2022, 44(2): 434-441. |
| Gao L, Li ZQ, Li K, et al. Advances in transgenic resistance to soybean mosaic virus disease [J]. Chin J Oil Crop Sci, 2022, 44(2): 434-441. | |
| [3] | 刘惠玲, 江炳玉, 张静, 等. 植物NAC转录因子的研究进展 [J]. 福建农林大学学报: 自然科学版, 2024, 53(6): 742-753. |
| Liu HL, Jiang BY, Zhang J, et al. Advances in study on plant NAC transcription factors [J]. J Fujian Agric For Univ: Nat Sci Ed, 2024, 53(6): 742-753. | |
| [4] | Todeschini AL, Georges A, Veitia RA. Transcription factors: specific DNA binding and specific gene regulation [J]. Trends Genet, 2014, 30(6): 211-219. |
| [5] | Baillo EH, Kimotho RN, Zhang ZB, et al. Transcription factors associated with abiotic and biotic stress tolerance and their potential for crops improvement [J]. Genes, 2019, 10(10): 771. |
| [6] | Zhang CQ, Grosic S, Whitham SA, et al. The requirement of multiple defense genes in soybean Rsv1-mediated extreme resistance to soybean mosaic virus [J]. Mol Plant Microbe Interact, 2012, 25(10): 1307-1313. |
| [7] | 钟晨丽, 兰胡娇, 王文絮, 等. GmWRKY33A正向调控大豆抗病性 [J]. 生物工程学报, 2024, 40(10): 3810-3822. |
| Zhong CL, Lan HJ, Wang WX, et al. GmWRKY33A positively regulates disease resistance insoybean (Glycine max) [J]. Chin J | |
| Biotechnol, 2024, 40(10): 3810-3822. | |
| [8] | 钟晨丽, 王文絮, 廖莉娜, 等. 沉默大豆GmWRKY33B基因导致大豆抗病性降低 [J]. 生物工程学报, 2024, 40(1): 163-176. |
| Zhong CL, Wang WX, Liao LN, et al. Silencing GmWRKY33B genes leads to reduced disease resistance in soybean [J]. Chin J Biotechnol, 2024, 40(1): 163-176. | |
| [9] | Ren QY, Jiang H, Xiang WY, et al. A MADS-box gene is involved in soybean resistance to multiple Soybean mosaic virus strains [J]. Crop J, 2022, 10(3): 802-808. |
| [10] | Li H, Liu JY, Yuan XX, et al. Comparative transcriptome analysis reveals key pathways and regulatory networks in early resistance of Glycine max to soybean mosaic virus [J]. Front Microbiol, 2023, 14: 1241076. |
| [11] | DeMers LC, Redekar NR, Kachroo A, et al. A transcriptional regulatory network of Rsv3-mediated extreme resistance against Soybean mosaic virus [J]. PLoS One, 2020, 15(4): e0231658. |
| [12] | Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis [J]. BMC Bioinformatics, 2008, 9: 559. |
| [13] | Zhu HH, Li RQ, Fang YY, et al. Weighted gene co-expression network analysis uncovers critical genes and pathways involved in soybean response to soybean mosaic virus [J]. Agronomy, 2024, 14(11): 2455. |
| [14] | Niu JP, Zhao J, Guo Q, et al. WGCNA reveals hub genes and key gene regulatory pathways of the response of soybean to infection by Soybean mosaic virus [J]. Genes, 2024, 15(5): 566. |
| [15] | Burke R, Schwarze J, Sherwood OL, et al. Stressed to death: the role of transcription factors in plant programmed cell death induced by abiotic and biotic stimuli [J]. Front Plant Sci, 2020, 11: 1235. |
| [16] | Masri R, Kiss E. The role of NAC genes in response to biotic stresses in plants [J]. Physiol Mol Plant Pathol, 2023, 126: 102034. |
| [17] | Viswanath KK, Kuo SY, Tu CW, et al. The role of plant transcription factors in the fight against plant viruses [J]. Int J Mol Sci, 2023, 24(9): 8433. |
| [18] | Wang X, Goregaoker SP, Culver JN. Interaction of the Tobacco mosaic virus replicase protein with a NAC domain transcription factor is associated with the suppression of systemic host defenses [J]. J Virol, 2009, 83(19): 9720-9730. |
| [19] | Xie Q, Sanz-Burgos AP, Guo H, et al. GRAB proteins, novel members of the NAC domain family, isolated by their interaction with a geminivirus protein [J]. Plant Mol Biol, 1999, 39(4): 647-656. |
| [20] | Huang Y, Li T, Xu ZS, et al. Six NAC transcription factors involved in response to TYLCV infection in resistant and susceptible tomato cultivars [J]. Plant Physiol Biochem, 2017, 120: 61-74. |
| [21] | Selth LA, Dogra SC, Saif Rasheed M, et al. A NAC domain protein interacts with tomato leaf curl virus replication accessory protein and enhances viral replication [J]. Plant Cell, 2005, 17(1): 311-325. |
| [22] | Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks [J]. Genome Res, 2003, 13(11): 2498-2504. |
| [23] | Rui R, Liu SC, Karthikeyan A, et al. Fine-mapping and identification of a novel locus Rsc15 underlying soybean resistance to soybean mosaic virus [J]. Theor Appl Genet, 2017, 130(11): 2395-2410. |
| [24] | Irsigler AST, Costa MDL, Zhang P, et al. Expression profiling on soybean leaves reveals integration of ER- and osmotic-stress pathways [J]. BMC Genomics, 2007, 8: 431. |
| [25] | Melo BP, Fraga OT, Silva JCF, et al. Revisiting the soybean GmNAC superfamily [J]. Front Plant Sci, 2018, 9: 1864. |
| [26] | Mendes GC, Reis PAB, Calil IP, et al. GmNAC30 and GmNAC81 integrate the endoplasmic reticulum stress- and osmotic stress-induced cell death responses through a vacuolar processing enzyme [J]. Proc Natl Acad Sci USA, 2013, 110(48): 19627-19632. |
| [27] | Melo BP, Lourenço-Tessutti IT, Fraga OT, et al. Contrasting roles of GmNAC065 and GmNAC085 in natural senescence, plant development, multiple stresses and cell death responses [J]. Sci Rep, 2021, 11(1): 11178. |
| [28] | Liu WJ, Mei ZX, Yu L, et al. The ABA-induced NAC transcription factor MdNAC1 interacts with a bZIP-type transcription factor to promote anthocyanin synthesis in red-fleshed apples [J]. Hortic Res, 2023, 10(5): uhad049. |
| [29] | Yu D, Wei W, Fan ZQ, et al. VabHLH137 promotes proanthocyanidin and anthocyanin biosynthesis and enhances resistance to Colletotrichum gloeosporioides in grapevine [J]. Hortic Res, 2022, 10(2): uhac261. |
| [30] | Zhong Q, Yu JT, Wu YD, et al. Rice transcription factor OsNAC2 maintains the homeostasis of immune responses to bacterial blight [J]. Plant Physiol, 2024, 195(1): 785-798. |
| [31] | Wang H, Bi Y, Gao YZ, et al. A Pathogen-Inducible rice nac transcription factor ONAC096 contributes to immunity against Magnaprothe oryzae and Xanthomonas oryzae pv. oryzae by direct binding to the promoters of OsRap2.6, OsWRKY62, and OsPAL1 [J]. Front Plant Sci, 2021, 12: 802758. |
| [32] | Gao SQ, Chen M, Xu ZS, et al. The soybean GmbZIP1 transcription factor enhances multiple abiotic stress tolerances in transgenic plants [J]. Plant Mol Biol, 2011, 75(6): 537-553. |
| [33] | 田蕊, 张华, 黄玫红, 等. 大豆抗旱遗传位点及候选基因发掘 [J]. 中国农业科技导报, 2023, 25(9): 69-82. |
| Tian R, Zhang H, Huang MH, et al. Mining of candidate genes and genetic loci conferring drought tolerance in soybean [J]. J Agric Sci Technol, 2023, 25(9): 69-82. | |
| [34] | Singh V, Roy S, Singh D, et al. Arabidopsis flowering locus D influences systemic-acquired-resistance- induced expression and histone modifications of WRKY genes [J]. J Biosci, 2014, 39(1): 119-126. |
| [35] | Nuruzzaman M, Sharoni AM, Kikuchi S. Roles of NAC transcription factors in the regulation of biotic and abiotic stress responses in plants [J]. Front Microbiol, 2013, 4: 248. |
| [36] | Freitas EO, Melo BP, Lourenço-Tessutti IT, et al. Identification and characterization of the GmRD26 soybean promoter in response to abiotic stresses: potential tool for biotechnological application [J]. BMC Biotechnol, 2019, 19(1): 79. |
| [37] | Pimenta MR, Silva PA, Mendes GC, et al. The stress-induced soybean NAC transcription factor GmNAC81 plays a positive role in developmentally programmed leaf senescence [J]. Plant Cell Physiol, 2016, 57(5): 1098-1114. |
| [38] | Wu YY, Liu XF, Fu BL, et al. Methyl jasmonate enhances ethylene synthesis in kiwifruit by inducing NAC genes that activate ACS1 [J]. J Agric Food Chem, 2020, 68(10): 3267-3276. |
| [39] | 曲硕, 刘芳, 孙浩文, 等. 大豆NAC转录因子生物信息学分析及GmNAC-1克隆和亚细胞定位 [J]. 大豆科学, 2024, 43(5): 523-538. |
| Qu S, Liu F, Sun HW, et al. Bioinformatics analysis of soybean NAC transcription factor and cloning and subcellular localization of GmNAC-1 [J]. Soybean Sci, 2024, 43(5): 523-538. |
| [1] | 李凯月, 邓晓霞, 殷缘, 杜亚彤, 徐元静, 王竞红, 于耸, 蔺吉祥. 蓖麻LEA基因家族的鉴定和铝胁迫响应分析[J]. 生物技术通报, 2025, 41(7): 128-138. |
| [2] | 韩燚, 侯昌林, 唐露, 孙璐, 谢晓东, 梁晨, 陈小强. 大麦HvERECTA基因的克隆及功能分析[J]. 生物技术通报, 2025, 41(7): 106-116. |
| [3] | 龚钰涵, 陈兰, 尚方慧子, 郝灵颖, 刘硕谦. 茶树TRB基因家族鉴定及表达模式分析[J]. 生物技术通报, 2025, 41(7): 214-225. |
| [4] | 赵强, 陈思宇, 彭方丽, 汪灿, 高杰, 周棱波, 张国兵, 姜昱雯, 邵明波. 间作与施氮对高粱根际土壤细菌多样性及功能的影响[J]. 生物技术通报, 2025, 41(6): 307-316. |
| [5] | 谭玉荣, 陈东亮, 杨守臻, 赖振光, 唐向民, 孙祖东, 曾维英. 大豆抗豆卷叶螟GmKTI1-like的功能研究[J]. 生物技术通报, 2025, 41(6): 99-108. |
| [6] | 王苗苗, 赵相龙, 王召明, 刘志鹏, 闫龙凤. 花苜蓿TCP基因家族的鉴定及其在干旱胁迫下的表达模式分析[J]. 生物技术通报, 2025, 41(6): 179-190. |
| [7] | 瞿美玲, 周思敏, 张惊宇, 何佳蔚, 朱佳源, 刘笑蓉, 童巧珍, 周日宝, 刘湘丹. 灰毡毛忍冬bHLH转录基因家族的鉴定与表达分析[J]. 生物技术通报, 2025, 41(6): 256-268. |
| [8] | 刘鑫, 王嘉雯, 李进伟, 牟策, 杨盼盼, 明军, 徐雷锋. 兰州百合三个LdBBXs基因的克隆与表达分析[J]. 生物技术通报, 2025, 41(5): 186-196. |
| [9] | 赵婧, 郭茜, 李睿琦, 雷滢炀, 岳爱琴, 赵晋忠, 殷丛丛, 杜维俊, 牛景萍. 大豆GmGST基因簇基因序列分析及诱导表达分析[J]. 生物技术通报, 2025, 41(5): 129-140. |
| [10] | 陈永旗, 李志文, 李鑫, 原若曦, 王春旭, 韩毅强, 高亚梅. 黑土区大豆根际土壤放线菌的分离与功能研究[J]. 生物技术通报, 2025, 41(5): 255-266. |
| [11] | 罗嗣芳, 张祖铭, 谢丽芳, 郭紫晶, 陈兆星, 杨月华, 严翔, 张洪铭. 山金柑GATA基因家族全基因组鉴定及在果实发育中的表达分析[J]. 生物技术通报, 2025, 41(5): 218-230. |
| [12] | 李志强, 罗正乾, 徐琳黎, 周国慧, 屈丝雨, 刘恩良, 顼东婷. 基于T2T基因组鉴定大豆R2R3-MYB基因家族及干旱和盐胁迫下的表达分析[J]. 生物技术通报, 2025, 41(5): 141-152. |
| [13] | 刘涛, 王志淇, 吴文博, 石文婷, 王超楠, 杜崇, 杨中敏. 马铃薯GRAM基因家族鉴定与表达分析[J]. 生物技术通报, 2025, 41(4): 145-155. |
| [14] | 孙天国, 衣兰, 秦旭洋, 乔梦雪, 谷新颖, 韩艺, 沙伟, 张梅娟, 马天意. 大白菜DABB基因家族的全基因组鉴定及盐碱胁迫下的表达分析[J]. 生物技术通报, 2025, 41(4): 156-165. |
| [15] | 王田田, 常雪瑞, 黄婉洋, 黄嘉欣, 苗如意, 梁燕平, 王静. 辣椒GASA基因家族的鉴定及分析[J]. 生物技术通报, 2025, 41(4): 166-175. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||