








生物技术通报 ›› 2026, Vol. 42 ›› Issue (4): 83-91.doi: 10.13560/j.cnki.biotech.bull.1985.2025-1073
李雅琦1,2( ), 孙萌1, 李秀丽1, 魏静娜1, 赵琳琳1, 赵云平1, 刘征辉1(
), 孙萌1, 李秀丽1, 魏静娜1, 赵琳琳1, 赵云平1, 刘征辉1( ), 苏蘩1(
), 苏蘩1( )
)
收稿日期:2025-10-10
出版日期:2026-02-09
发布日期:2026-02-09
通讯作者:
刘征辉,男,硕士,副研究员,研究方向 :农产品质量安全;E-mail: liuzhenghui_2025@126.com作者简介:李雅琦,女,硕士研究生,研究方向 :植物病原检测;E-mail: 1072636135@qq.com基金资助:
LI Ya-qi1,2( ), SUN Meng1, LI Xiu-li1, WEI Jing-na1, ZHAO Lin-lin1, ZHAO Yun-ping1, LIU Zheng-hui1(
), SUN Meng1, LI Xiu-li1, WEI Jing-na1, ZHAO Lin-lin1, ZHAO Yun-ping1, LIU Zheng-hui1( ), SU Fan1(
), SU Fan1( )
)
Received:2025-10-10
Published:2026-02-09
Online:2026-02-09
摘要:
目的 现有CRISPR/Cas12a检测体系多沿用限制性内切酶的通用反应缓冲液,但该类缓冲体系未充分考虑其在Cas12a荧光信号释放中的适配性,限制了检测灵敏度并增加了应用成本。为此,本研究旨在构建一种高适配性、低成本且适用于多种Cas12a蛋白的荧光优化反应体系,以显著提升核酸检测性能。 方法 通过荧光检测器定量分析与可视化检测,系统评估pH值(7.3-7.9,25 ℃)、Tris-HCl(5-50 mmol/L)、Ca2+(0.1-1 mmol/L)及Mg2+(10-30 mmol/L)对Cas12a荧光信号的影响。在此基础上,构建不含抗氧化剂及蛋白稳定剂的缓冲液CasRB,并与NEB系列商品缓冲液进行性能比较,同时验证其在FnCas12a、AsCas12a和LbCas12a三种蛋白体系中的通用性。 结果 优化后的CasRB(10 mmol/L Tris-HCl、0.1 mmol/L CaCl₂、20 mmol/L MgCl₂,pH 7.9,25 ℃)在不含高价组分(DTT和蛋白稳定剂)的条件下,与NEB系列商品缓冲液相比,成本降低超过99.9%;荧光信噪比提升10倍以上,显著增强裸眼判读效果,且表现出良好的跨菌源Cas12a蛋白适用性。 结论 本研究开发的CasRB缓冲液兼具成本优势和性能优势,解决了通用缓冲液在Cas12a荧光检测系统适配性不足的问题。
李雅琦, 孙萌, 李秀丽, 魏静娜, 赵琳琳, 赵云平, 刘征辉, 苏蘩. 多种Cas12a蛋白普适性的高性能低成本荧光检测缓冲体系优化研究[J]. 生物技术通报, 2026, 42(4): 83-91.
LI Ya-qi, SUN Meng, LI Xiu-li, WEI Jing-na, ZHAO Lin-lin, ZHAO Yun-ping, LIU Zheng-hui, SU Fan. Optimization of a High-performance and Low-cost Fluorescence Detection Buffer with Broad Compatibility across Cas12a Orthologs[J]. Biotechnology Bulletin, 2026, 42(4): 83-91.
| 名称 Name | 序列 Sequences(5'-3') |
|---|---|
| dsDNA activator | CTCGCGAGTCCCAACACCAAGCTGGGCTTGAGGGTTGAAATGACGCTCGAACAGGCATGCCCGCCAGAATACTGGCGGGCGCAATGTGCGTTCAAAGATTCGATGATTCACTGAATTCTGCAATTCACATTACTTATCGCATTTT GCTGCGTTCTTCATCGATGC CAGAACCAAGAGATCCGTTGTTGAAAGTTTTGATTTATTTATGGTTTTACTCAGAAGTTACATATAGAAACAGAGTTTAGGGGTCCTCTGGCGGGCCGTCCCGTTTTACCGGGAGCGGGCTGATCCGCCGAGGCA |
| crRNA-F | TAATACGACTCACTATAGGGAATTTCTACTGTTGTAGAT |
| crRNA-R | GCATCGATGAAGAACGCAGCATCTACAACAGTAGAAATT |
| crRNA |
表1 Cas12a体系所用dsDNA激活片段、crRNA序列及引物信息
Table 1 dsDNA activator fragments, crRNA sequences, and primer information used in the Cas12a system
| 名称 Name | 序列 Sequences(5'-3') |
|---|---|
| dsDNA activator | CTCGCGAGTCCCAACACCAAGCTGGGCTTGAGGGTTGAAATGACGCTCGAACAGGCATGCCCGCCAGAATACTGGCGGGCGCAATGTGCGTTCAAAGATTCGATGATTCACTGAATTCTGCAATTCACATTACTTATCGCATTTT GCTGCGTTCTTCATCGATGC CAGAACCAAGAGATCCGTTGTTGAAAGTTTTGATTTATTTATGGTTTTACTCAGAAGTTACATATAGAAACAGAGTTTAGGGGTCCTCTGGCGGGCCGTCCCGTTTTACCGGGAGCGGGCTGATCCGCCGAGGCA |
| crRNA-F | TAATACGACTCACTATAGGGAATTTCTACTGTTGTAGAT |
| crRNA-R | GCATCGATGAAGAACGCAGCATCTACAACAGTAGAAATT |
| crRNA |
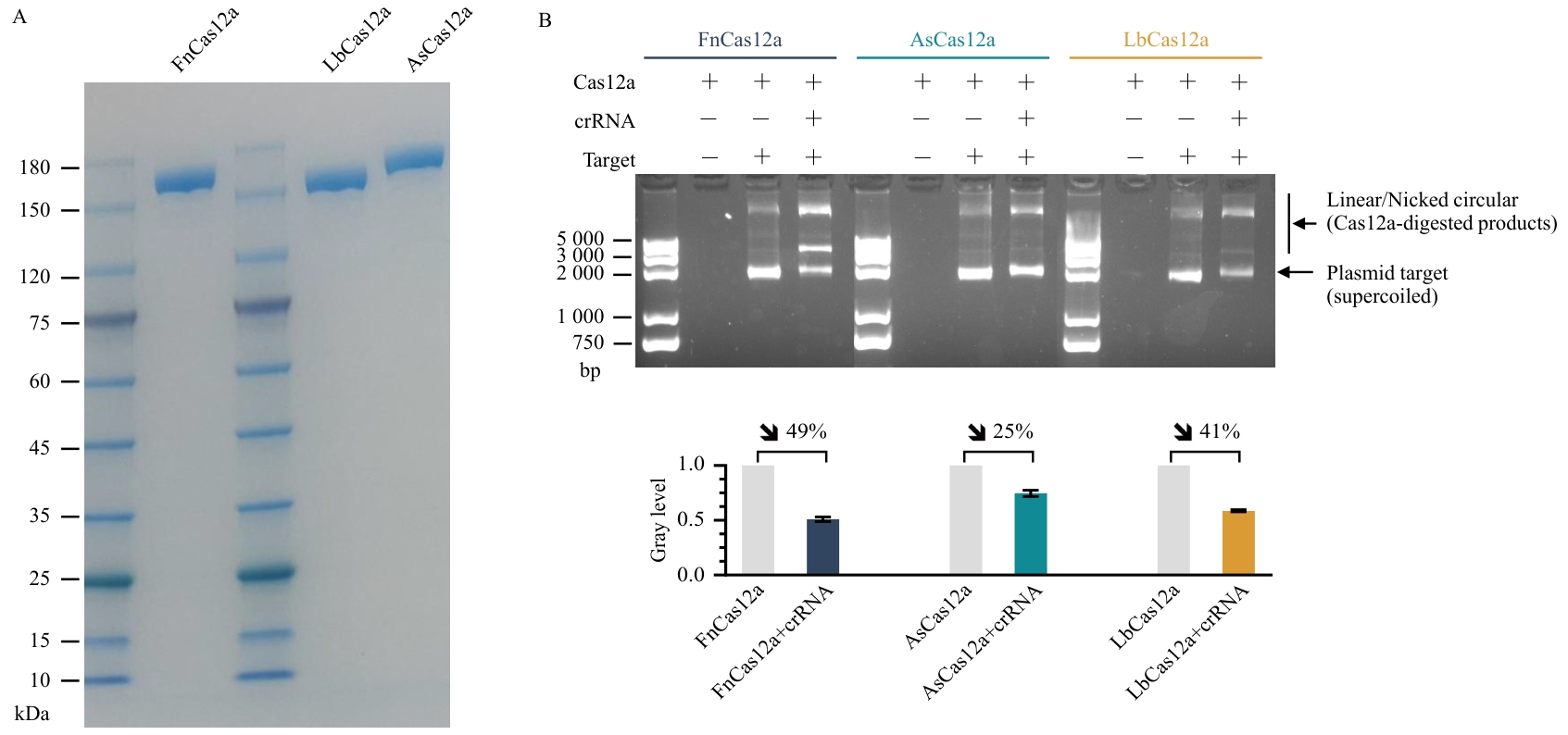
图1 Cas12a蛋白的表达纯化及体外酶切活性分析A:Cas12a重组蛋白的SDS-PAGE分析;B:三种Cas12a蛋白的体外DNA酶切活性检测;上图:琼脂糖凝胶电泳分析crRNA引导下各Cas12a蛋白对质粒DNA底物的切割情况。下图:对质粒靶标的灰度定量分析结果(n=3),数据以均值±标准误表示。
Fig. 1 Expression, purification, and in vitro cleavage activity analysis of Cas12a proteinsA: SDS-PAGE analysis of recombinant Cas12a proteins; B: In vitro DNA cleavage assays of three Cas12a proteins; Top: Agarose gel electrophoresis showing crRNA-guided cleavage of plasmid DNA substrates by each Cas12a protein. Bottom: Quantitative grayscale analysis of plasmid targets (n=3). Data are presented as mean ± SEM.

图2 关键反应条件对CRISPR/Cas12a荧光生物传感器性能的影响A:pH值的影响;B:Tris-HCl浓度的影响;C:Ca²⁺浓度的影响;D:Mg²⁺浓度的影响。每个条件均展示:荧光信号实时检测曲线和信噪比统计分析(n=3,数据以均值±标准误表示;显著性差异采用GraphPad Prism 7软件进行Kruskal-Wallis检验并结合Dunn’s多重比较检验分析,以不同字母标注,P<0.05),以及470 nm蓝光照射下的肉眼观测结果。实验中设置阳性样本(+,含质粒靶标)与阴性对照(-,不含质粒靶标),用于评估体系的有效激活信号与背景信号。下同
Fig. 2 Effect of key reaction conditions for the CRISPR/Cas12a fluorescence biosensor performanceA: Effect of pH; B: Effect of Tris-HCl concentration; C: Effect of Ca²⁺ concentration; D: Effect of Mg²⁺ concentration. Each condition shows real-time fluorescence detection curves and signal-to-noise ratio analysis (n=3, data presented as mean ± SEM; significant differences determined using GraphPad Prism 7 with Kruskal-Wallis test followed by Dunn’s multiple comparisons test, indicated by different letters, P<0.05), as well as visual observation under 470 nm blue light illumination. Positive samples (+, with plasmid target) and negative controls (-, without plasmid target) were included to evaluate effective signal activation and background fluorescence. The same below
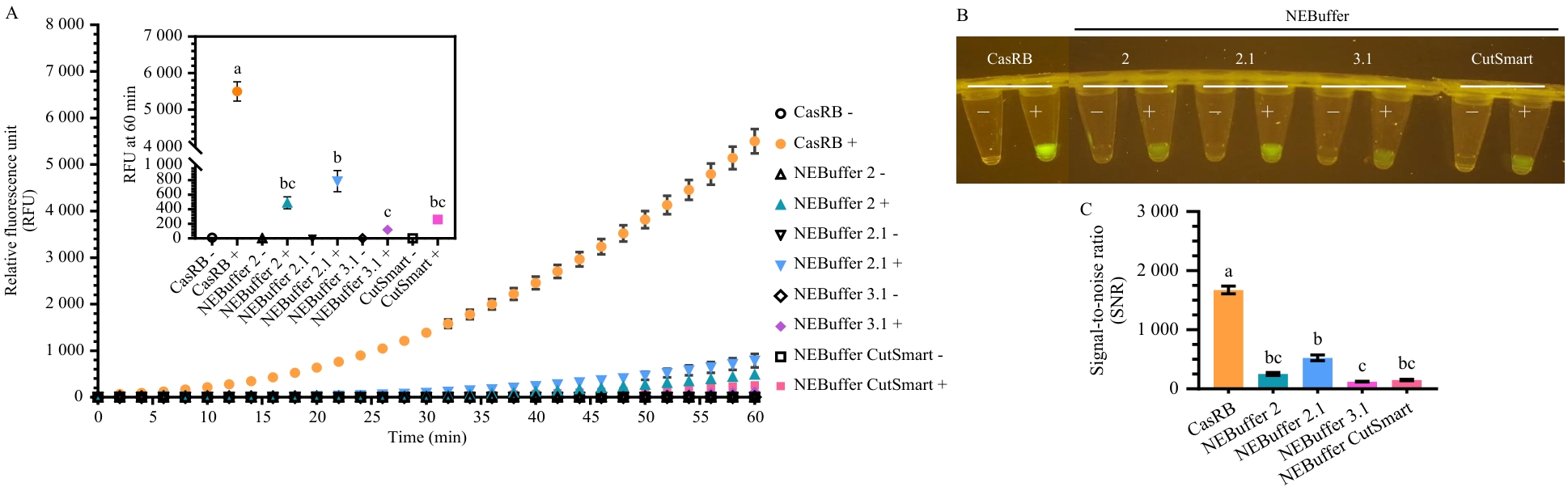
图3 CasRB与商品化缓冲液在CRISPR/Cas12a荧光检测体系中的性能对比A:荧光信号实时检测曲线;B:470 nm蓝光照射下的肉眼观测结果;C:荧光信号信噪比
Fig. 3 Performance comparison between CasRB and commercial buffers in the CRISPR/Cas12a fluorescence detection systemA: Real-time fluorescence detection curves; B: Visual observation under 470 nm blue light illumi nation; C: Signal-to-noise ratio of fluorescence detection
| 缓冲液体系Buffer | 信噪比(均值±标准差) Signal-to-noise ratio (Mean ± SD) | 相对信噪比 Relative SNR (CasRB=1) | 单反应成本估算(分) Estimated cost per reaction (CNY cent) | 相对成本 Relative cost (CasRB=1) |
|---|---|---|---|---|
| CasRB | 1 672.8±110.9 | 1.00 | 0.015a | 1 |
| NEBuffer 2 | 253.3±43.6 | 0.15 | 24.54b | 1 636 |
| NEBuffer 2.1 | 525.4±82.8 | 0.31 | 24.54b | 1 636 |
| NEBuffer 3.1 | 122.0±11.4 | 0.07 | 24.54b | 1 636 |
| NEBuffer CutSmart | 149.9±18.2 | 0.09 | 24.54b | 1 636 |
表2 CasRB与商品化缓冲液在60 min终点的信噪比(SNR)与成本对比
Table 2 Comparison of signal-to-noise ratio (SNR) and estimated cost between CasRB and commercial buffers at the 60 min endpoint
| 缓冲液体系Buffer | 信噪比(均值±标准差) Signal-to-noise ratio (Mean ± SD) | 相对信噪比 Relative SNR (CasRB=1) | 单反应成本估算(分) Estimated cost per reaction (CNY cent) | 相对成本 Relative cost (CasRB=1) |
|---|---|---|---|---|
| CasRB | 1 672.8±110.9 | 1.00 | 0.015a | 1 |
| NEBuffer 2 | 253.3±43.6 | 0.15 | 24.54b | 1 636 |
| NEBuffer 2.1 | 525.4±82.8 | 0.31 | 24.54b | 1 636 |
| NEBuffer 3.1 | 122.0±11.4 | 0.07 | 24.54b | 1 636 |
| NEBuffer CutSmart | 149.9±18.2 | 0.09 | 24.54b | 1 636 |
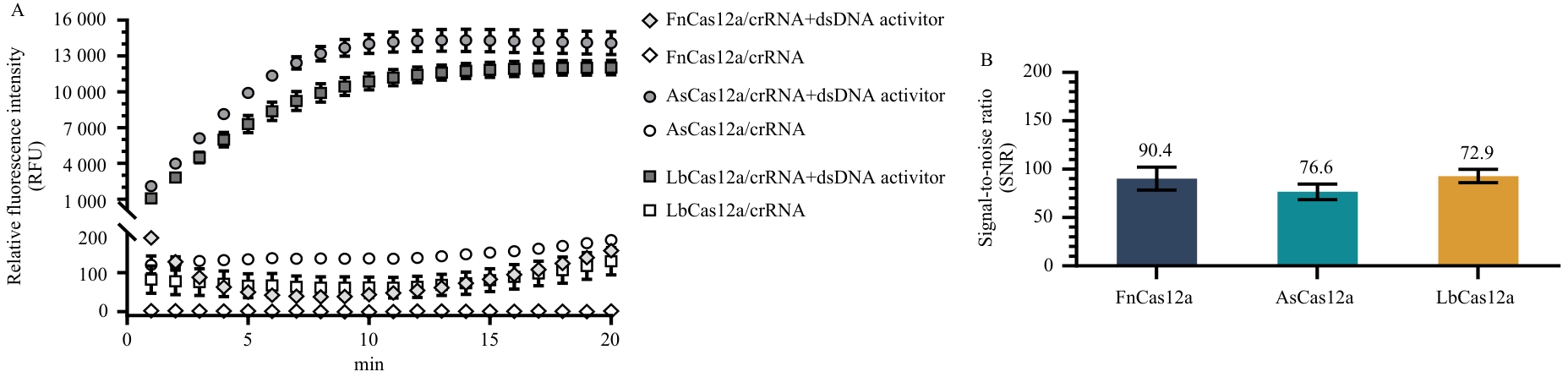
图4 CasRB对不同Cas12a蛋白的适用性评价A:荧光信号实时检测曲线;B:反应20分钟时荧光信号信噪比。
Fig. 4 Evaluation of the applicability of CasRB to different Cas12a enzymesA: Real-time fluorescence detection curves; B: Signal-to-noise ratio of fluorescence detection at 20 min.
| [1] | Chen JS, Ma E, Harrington LB, et al. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity [J]. Science, 2018, 360(6387): 436-439. |
| [2] | Xin XL, Su J, Cui HR, et al. Recent advances in clustered regularly interspaced short palindromic repeats/CRISPR-associated proteins system-based biosensors [J]. Biosensors, 2025, 15(3): 155. |
| [3] | Pan LY, Jiang WW, Deng F, et al. Signal-amplification strategy and advances in electrochemistry-based CRISPR/Cas12 biosensing [J]. Chem Eng J, 2025, 508: 161110. |
| [4] | Swarts DC, Jinek M. Mechanistic insights into the cis- and trans-acting DNase activities of Cas12a [J]. Mol Cell, 2019, 73(3): 589-600.e4. |
| [5] | Son H, Park J, Choi YH, et al. Exploring the dynamic nature of divalent metal ions involved in DNA cleavage by CRISPR-Cas12a [J]. Chem Commun, 2022, 58(12): 1978-1981. |
| [6] | Song Y, Xu YW, Wang RR, et al. CRISPR-Cas12a-based nanoparticle biosensor for detection of pathogenic bacteria in food [J]. Microchem J, 2024, 207: 111813. |
| [7] | Ma YJ, Wei HJ, Wang YX, et al. Efficient magnetic enrichment cascade single-step RPA-CRISPR/Cas12a assay for rapid and ultrasensitive detection of Staphylococcus aureus in food samples [J]. J Hazard Mater, 2024, 465: 133494. |
| [8] | 田道明, 周子萱, 杨迎澳, 等. CRISPR-Cas12a介导的适配体荧光传感器快速检测金黄色葡萄球菌 [J]. 中国医药科学, 2024(21): 139-143. |
| Tian DM, Zhou ZX, Yang YA, et al. Rapid detection of Staphylococcus aureus by aptamer fluorescence sensor mediated by CRISPR-Cas12a [J]. China Medicine and Pharmacy, 2024(21): 139-143. | |
| [9] | He W, Liao K, Li RX, et al. Development of a CRISPR/Cas12a-based fluorescent detection method of senecavirus A [J]. BMC Vet Res, 2024, 20(1): 258. |
| [10] | Cao ZZ, Li Z, Jin KK, et al. A rapid and visual detection method for porcine delta coronavirus with single-copy sensitivity based on the CRISPR/Cas12a assay [J]. J Integr Agric, 2025 |
| [11] | 热则古丽·艾科拜尔, 张亚平, 刘浩然, 等. 基于RAA-CRISPR/Cas12a建立牛病毒性腹泻病毒的可视化快速检测方法及其初步应用 [J]. 中国兽医科学, 2025(3): 321-329. |
| Reze Guli A, Zhang YP, Liu HR, et al. Establishment of a visual rapid detection method for bovine viral diarrhea virus based on RAA-CRISPR/Cas12a and its preliminary application [J]. Chinese Veterinary Science, 2025(3): 321-329. | |
| [12] | Shashank PR, Parker BM, Rananaware SR, et al. CRISPR-based diagnostics detects invasive insect pests [J]. Mol Ecol Resour, 2024, 24(1): e13881. |
| [13] | Mu KQ, Ren XJ, Yang H, et al. CRISPR-Cas12a-based diagnostics of wheat fungal diseases [J]. J Agric Food Chem, 2022, 70(23): 7240-7247. |
| [14] | Fang YX, Liu LJ, Zhao WY, et al. Rapid and sensitive detection of Verticillium dahliae from soil using LAMP-CRISPR/Cas12a technology [J]. Int J Mol Sci, 2024, 25(10): 5185. |
| [15] | Guo YF, Tan JJ, Jiao BB, et al. Establishment of a rapid detection system for Phytophthora syringae based on RPA/CRISPR-Cas12a [J]. Crop Prot, 2025, 190: 107106. |
| [16] | Nguyen LT, Rananaware SR, Pizzano BLM, et al. Clinical validation of engineered CRISPR/Cas12a for rapid SARS-CoV-2 detection [J]. Commun Med, 2022, 2: 7. |
| [17] | Dong JJ, Wang SJ, Xu WX, et al. Understanding the stability landscape of LbCas12a by deep analysis of stabilizing mutations and mutation combinations [J]. Protein Sci, 2025, 34(9): e70280. |
| [18] | Tong XH, Li TL, Zhang K, et al. Structure-guided design of Cas12a variants improves detection of nucleic acids [J]. Cell Insight, 2025, 4(2): 100228. |
| [19] | Hwang I, Song YH, Lee S. Enhanced trans-cleavage activity using CRISPR-Cas12a variant designed to reduce steric inhibition by cis-cleavage products [J]. Biosens Bioelectron, 2025, 267: 116859. |
| [20] | Nguyen LT, Smith BM, Jain PK. Enhancement of trans-cleavage activity of Cas12a with engineered crRNA enables amplified nucleic acid detection [J]. Nat Commun, 2020, 11: 4906. |
| [21] | Li YY, University CM, Hu QF, et al. CrRNA conformation-engineered CRISPR-Cas12a system for robust and ultrasensitive nucleic acid detection [J]. Anal Chem, 2025, 97(6): 3617-3624. |
| [22] | Cheng ZH, Luo XY, Yu SS, et al. Tunable control of Cas12 activity promotes universal and fast one-pot nucleic acid detection [J]. Nat Commun, 2025, 16: 1166. |
| [23] | Son H, Park J, Hwang I, et al. Mg2+-dependent conformational rearrangements of CRISPR-Cas12a R-loop complex are mandatory for complete double-stranded DNA cleavage [J]. Proc Natl Acad Sci U S A, 2021, 118(49): e2113747118. |
| [24] | Wang D, Ma DJ, Han JX, et al. CRISPR RNA array-guided multisite cleavage for gene disruption by Cas9 and Cpf1 [J]. ChemBioChem, 2018, 19(20): 2195-2205. |
| [25] | Su F, Wang S, Gao W, et al. Visual detection of multiple Fusarium species via RAA-CRISPR/Cas12a dual fluorescence-lateral flow assay [J]. J Future Foods, 2025 |
| [26] | Chen JL, Huang YE, Xiao B, et al. Development of a RPA-CRISPR-Cas12a assay for rapid, simple, and sensitive detection of Mycoplasma hominis [J]. Front Microbiol, 2022, 13: 842415. |
| [27] | Pan JF, Deng F, Liu Z, et al. Construction of molecular logic gates using heavy metal ions as inputs based on catalytic hairpin assembly and CRISPR-Cas12a [J]. Talanta, 2023, 255: 124210. |
| [28] | Nguyen GT, Schelling MA, Raju A, et al. CRISPR-Cas12a exhibits metal-dependent specificity switching [J]. Nucleic Acids Res, 2024, 52(16): 9343-9359. |
| [29] | Zhu FX, Yu H, Zhao Q. CRISPR/Cas12a-amplified aptamer switch microplate assay for small molecules [J]. Anal Chem, 2024, 96(17): 6853-6859. |
| [30] | Zhang YD, He BS, Zhang YR, et al. An aptasensor based on CRISPR/cas12a-mediated and Mn2+-assisted DNA motor cascade signalling amplification strategy for the detection of T-2 toxin [J]. Sens Actuat B Chem, 2025, 426: 137049. |
| [31] | Liu Y, Liu XY, Wei DY, et al. CoHIT: a one-pot ultrasensitive ERA-CRISPR system for detecting multiple same-site indels [J]. Nat Commun, 2024, 15: 5014. |
| [1] | 霍贯中, 张欣濡, 田士军, 李君. CRISPR/Cas12a基因编辑技术在植物中的研究进展[J]. 生物技术通报, 2025, 41(6): 1-11. |
| [2] | 高畅, 庄添驰, 李宁, 刘云, 顾鹏飞, 赵昕怡, 季明辉. RPA-CRISPR/Cas12a结合重力驱动微流控芯片的MTB快检方法的建立[J]. 生物技术通报, 2025, 41(5): 62-69. |
| [3] | 佟德利, 张馨, 陈佳庆, 贺海升. 蓝莓内生细菌的分离及其对上海青铝胁迫的缓解作用[J]. 生物技术通报, 2025, 41(4): 302-311. |
| [4] | 宋奋奋, 段艳雪, 桑愉, 王继朋, 彭锐, 孙年喜, 李勇. 患病和健康羊肚菌菌丝际土壤微生物群落特征[J]. 生物技术通报, 2025, 41(4): 323-334. |
| [5] | 潘忠飞, 尹倩, 马容, 熊欢, 董文统, 邹锋. 外源溶磷菌促进油茶养分吸收的机制[J]. 生物技术通报, 2025, 41(10): 292-302. |
| [6] | 姚雪春, 李磊, 王志贤, 盛长忠, ZHOU Zeqi, TAN Cherie S. 基于CRISPR-Cas12a技术的呼吸道合胞病毒检测方法的建立[J]. 生物技术通报, 2025, 41(1): 103-109. |
| [7] | 陈墨岩, 祝诚. 基于CRISPR/Cas12a的生物传感平台的机制研究及应用[J]. 生物技术通报, 2024, 40(7): 90-98. |
| [8] | 李雪, 李容欧, 孔美懿, 黄磊. 解淀粉芽孢杆菌SQ-2对水稻的促生作用[J]. 生物技术通报, 2024, 40(2): 109-119. |
| [9] | 张林林, 沈虎生, 杨冰, 何梦菡, 朴凤植, 申顺善. 生防细菌HK11-9对黄瓜棒孢叶斑病的防病能力及其鉴定[J]. 生物技术通报, 2023, 39(12): 209-218. |
| [10] | 胡海洋, 应婉琴, 何军, 吕芷贤, 谢小平, 邓仲良. 酶促重组等温扩增实时荧光法快速检测肺炎支原体方法的建立及应用[J]. 生物技术通报, 2022, 38(9): 264-270. |
| [11] | 赵忠娟, 杨凯, 扈进冬, 魏艳丽, 李玲, 徐维生, 李纪顺. 盐胁迫条件下哈茨木霉ST02对椒样薄荷生长及根区土壤理化性质的影响[J]. 生物技术通报, 2022, 38(7): 224-235. |
| [12] | 王小琴, 黄银萍, 王蔚倩, 吴萍, 全舒. 含非天然氨基酸定点突变的MLL3SET蛋白表达与纯化[J]. 生物技术通报, 2022, 38(3): 194-202. |
| [13] | 贾海红, 李冰清. 超氧化物歧化酶翻译后修饰的研究进展[J]. 生物技术通报, 2022, 38(2): 237-244. |
| [14] | 武杞蔓, 田诗涵, 李昀烨, 潘英杰, 张颖. 微生物菌肥对设施黄瓜生长、产量及品质的影响[J]. 生物技术通报, 2022, 38(1): 125-131. |
| [15] | 胡秀文, 刘华, 王宇, 唐雪明, 王金斌, 曾海娟, 蒋玮, 李红. CRISPR-Cas系统在核酸检测中的应用研究[J]. 生物技术通报, 2021, 37(9): 266-273. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||