








生物技术通报 ›› 2022, Vol. 38 ›› Issue (11): 258-268.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1617
王小桃( ), 邹杭, 吴怡, 向省维, 吕华, 刘超兰, 林家富, 王欣荣, 褚以文, 宋涛(
), 邹杭, 吴怡, 向省维, 吕华, 刘超兰, 林家富, 王欣荣, 褚以文, 宋涛( )
)
收稿日期:2021-12-30
出版日期:2022-11-26
发布日期:2022-12-01
作者简介:王小桃,女,硕士研究生,研究方向:微生物药物;E-mail:基金资助:
WANG Xiao-tao( ), ZOU Hang, WU Yi, XIANG Shen-wei, LV Hua, LIU Chao-lan, LIN Jia-fu, WANG Xin-rong, CHU Yi-wen, SONG Tao(
), ZOU Hang, WU Yi, XIANG Shen-wei, LV Hua, LIU Chao-lan, LIN Jia-fu, WANG Xin-rong, CHU Yi-wen, SONG Tao( )
)
Received:2021-12-30
Published:2022-11-26
Online:2022-12-01
摘要:
琼胶寡糖具有抗氧化、抗肿瘤和调节肠道菌群等多种生物活性,而微生物来源的琼胶酶是酶法制备琼胶寡糖的重要工具酶。目前报道的琼胶酶数量较少,而具有优良酶学特性的琼胶酶数量更少,极大阻碍了酶法制备琼胶寡糖的工艺开发进程。因此有必要发掘更多微生物来源的新颖琼胶酶。从副居冰菌属Paraglaciecola hydrolytica细菌基因组中挖掘到一个新颖琼胶酶基因aga2,构建至表达载体 pET28a(+),并在大肠杆菌 BL21(DE3)中进行表达;通过镍金属亲和层析纯化蛋白并探究温度、pH、金属离子、NaCl浓度对Aga2活性的影响;采用13C核磁共振、薄层色谱和基质辅助激光解吸飞行时间质谱分析酶解产物。Aga2与已知琼胶酶的最高相似度为53.7%。同时Aga2在IPTG(Isopropyl-beta-D-thiogalactopyranoside)浓度为90 μmol/L,20℃下诱导9 h时,可溶性表达量最高。纯化的Aga2最适反应温度为50℃,且40℃孵育3 h后仍保持72.9%的相对酶活力,具有较好的温度稳定性。Aga2的最适pH为6.0,在不同pH(4-9)下放置5 h后,仍保持62.6%以上的相对酶活力,具有较好的pH稳定性。Aga2在NaCl浓度为2.5 mol/L时仍保持78%的相对酶活力,具有较强的盐耐受性。同时Aga2对Ni2+、Ca2+、Ba2+、K+、Mg2+、Zn2+、EDTA、DTT、Urea、SDS、TritionX-100有耐受性。薄层色谱结果表明,该酶属于内切型β琼胶酶,产物为新琼四糖和新琼六糖。Aga2具有温度和pH稳定性、盐耐受性和重金属离子耐受性,具有良好的工业应用前景。
王小桃, 邹杭, 吴怡, 向省维, 吕华, 刘超兰, 林家富, 王欣荣, 褚以文, 宋涛. Paraglaciecola hydrolytica中新型β-琼胶酶Aga2的异源表达及酶学性质分析[J]. 生物技术通报, 2022, 38(11): 258-268.
WANG Xiao-tao, ZOU Hang, WU Yi, XIANG Shen-wei, LV Hua, LIU Chao-lan, LIN Jia-fu, WANG Xin-rong, CHU Yi-wen, SONG Tao. Heterologous Expression and Enzymatic Properties Analysis of Novel β-agarase Aga2 from Paraglaciecola hydrolytica[J]. Biotechnology Bulletin, 2022, 38(11): 258-268.
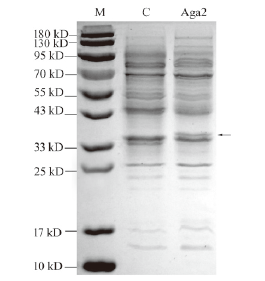
图3 琼胶酶Aga2的总蛋白电泳图 M:蛋白 marker;C:空载对照;上样量为 5-10 μg
Fig. 3 SDS-PAGE analysis of agarases Aga2 M:Protein marker. C:Empty control;the loading amount is 5-10 μg

图4 IPTG浓度、诱导温度和诱导时间对Aga2的影响 M:蛋白marker;A:1-7:表示IPTG的浓度为0 μmol/L、30 μmol/L、60 μmol/L、90 μmol/L、120 μmol/L、150 μmol/L、180 μmol/L时的蛋白样品;C:1-5:诱导温度为16℃、20℃、24℃、28℃、32℃时的蛋白样品;E:1-6:诱导时间为0 h、3 h、6 h、9 h、12 h、15 h时的蛋白样品
Fig.4 Effects of IPTG,temperature and induction time concentration on Aga2 M:Protein marker. A:1-7:protein samples when the concentration of IPTG is 0,30,60,90,120,150和 180 μmol/L,respectively. C:1-5:protein samples when the induction temperatures is 16℃,20℃,24℃,28℃,and 32℃. E:1-6:protein samples when the induction times is 0,3,6,9,12 and 15 h
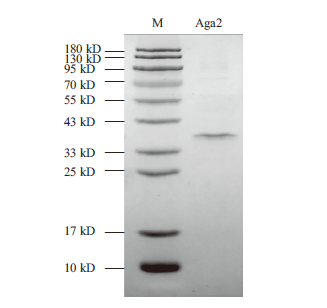
图5 镍金属亲和层析纯化后的Aga2蛋白电泳图 M:蛋白marker;Aga2:纯化的Aga2;上样量为1-5 μg
Fig. 5 SDS-PAGE analysis of purified Aga2 after Ni affi-nity chromatography M:Protein marker. Aga2:Purified Aga2. The loading amount is 1-5 μg
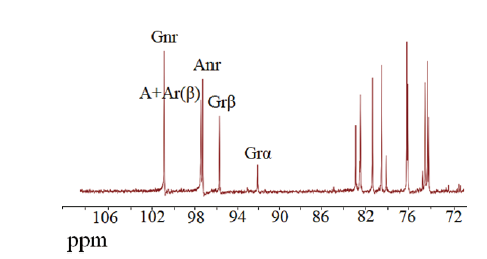
图12 Aga2降解琼胶生成底物的碳核磁共振分析 G和A:D-半乳糖和3,6-L-内醚半乳糖;r和nr:还原端和非还原端;α和β:α碳原子和β碳原子
Fig.12 CNMR analysis of agar hydrolysis product catalyz-ed by Aga2 G and A:D-galactose and 3,6-Anhydro-L-galactose;r and nr:reducing and non-reducing ends;α and β:α carbon atoms and β carbon atoms
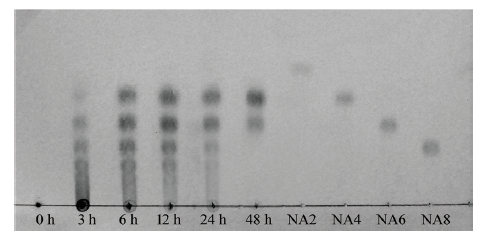
图13 琼胶酶 Aga2降解琼脂糖不同时间点的TLC图 NA8:新琼八糖标准品;NA6:新琼六糖标准品;NA4:新琼四糖标准品;NA2表示新琼二糖标准品
Fig. 13 TLC analysis of hydrolysis product by Aga2 during different time points NA8:Neoagarooctaose standard;NA6:neoagarohexaose standard;NA4:neoagarotetraose standard;NA2:neoagarobiose standard
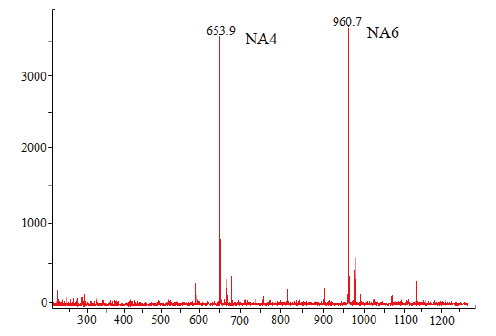
图14 Aga2降解琼胶生成寡糖的MALDI-TOF-MS结果示意图 NA6:新琼六糖标准品;NA4:新琼四糖标准品
Fig. 14 MALDI-TOF-MS analysis of oligosaccharides from agar hydrolysis catalyzed by Aga2 NA6:Neoagarohexaose standard;NA4:neoagarotetraose standard
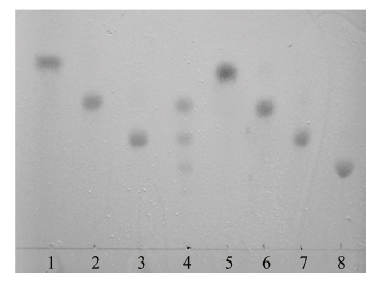
图15 Aga2降解不同长度寡糖的TLC结果分析 1-4:Aga2分别和新琼二糖、新琼四糖、新琼六糖、新琼八糖混合反应的产物;5-8分别为:新琼二糖标准品、新琼四糖标准品、新琼六糖标准品、新琼八糖标准品
Fig. 15 TLC analysis of different oligosaccharide hydroly-sis product catalyzed by Aga2 1-4:Mixed reaction products of Aga2 and neoagarobiose,Aga2 and neoagarote-traose,Aga2 and neoagarohexaose,Aga2 and neoagarooctaose,respectively. 5-8:Neoagarobiose standard,neoagarotetraose standard,neoagarohexaose standard,and neoagarooctaose standard,respectively
| [1] |
Liu N, Yang M, Mao XZ, et al. Molecular cloning and expression of a new α-neoagarobiose hydrolase from Agarivorans gilvus WH0801 and enzymatic production of 3, 6-anhydro-l-galactose[J]. Biotechnol Appl Biochem, 2016, 63(2):230-237.
doi: 10.1002/bab.1363 URL |
| [2] |
Kobayashi R, Takisada M, Suzuki T, et al. Neoagarobiose as a novel moisturizer with whitening effect[J]. Biosci Biotechnol Biochem, 1997, 61(1):162-163.
doi: 10.1271/bbb.61.162 URL |
| [3] | 李海新, 梁家铭, 李蓉, 等. 琼胶寡糖的抗氧化活性研究[J]. 广州化工, 2018, 46(20):66-68. |
| Li HX, Liang JM, Li R, et al. Study on antioxidant activity of agar-oligosaccharide[J]. Guangzhou Chem Ind, 2018, 46(20):66-68. | |
| [4] |
Ariga O, Okamoto N, Harimoto N, et al. Purification and characterization of α-neoagarooligosaccharide hydrolase from Cellvibrio sp. OA-2007[J]. J Microbiol Biotechnol, 2014, 24(1):48-51.
doi: 10.4014/jmb.1307.07018 URL |
| [5] |
Jin M, Liu HF, Hou YP, et al. Preparation, characterization and alcoholic liver injury protective effects of algal oligosaccharides from Gracilaria lemaneiformis[J]. Food Res Int, 2017, 100(Pt 2):186-195.
doi: 10.1016/j.foodres.2017.08.032 URL |
| [6] |
Higashimura Y, Naito Y, Takagi T, et al. Protective effect of agaro-oligosaccharides on gut dysbiosis and colon tumorigenesis in high-fat diet-fed mice[J]. Am J Physiol Gastrointest Liver Physiol, 2016, 310(6):G367-G375.
doi: 10.1152/ajpgi.00324.2015 URL |
| [7] |
Liang SS, Chen YP, Chen YH, et al. Characterization and overexpression of a novel β-agarase from Thalassomonas Agarivorans[J]. J Appl Microbiol, 2014, 116(3):563-572.
doi: 10.1111/jam.12389 pmid: 24206167 |
| [8] |
Zhang WB, Xu JN, Liu D, et al. Characterization of an α-agarase from Thalassomonas sp. LD5 and its hydrolysate[J]. Appl Microbiol Biotechnol, 2018, 102(5):2203-2212.
doi: 10.1007/s00253-018-8762-6 URL |
| [9] |
Fu XT, Kim SM. Agarase:review of major sources, categories, purification method, enzyme characteristics and applications[J]. Mar Drugs, 2010, 8(1):200-218.
doi: 10.3390/md8010200 URL |
| [10] |
Jahromi ST, Barzkar N. Future direction in marine bacterial agarases for industrial applications[J]. Appl Microbiol Biotechnol, 2018, 102(16):6847-6863.
doi: 10.1007/s00253-018-9156-5 pmid: 29909571 |
| [11] |
Ohta Y, Hatada Y, Nogi Y, et al. Enzymatic properties and nucleotide and amino acid sequences of a thermostable β-agarase from a novel species of deep-sea Microbulbifer[J]. Appl Microbiol Biotechnol, 2004, 64(4):505-514.
pmid: 15088129 |
| [12] |
Hsu PH, Wei CH, Lu WJ, et al. Extracellular production of a novel endo-β-agarase AgaA from Pseudomonas vesicularis MA103 that cleaves agarose into neoagarotetraose and neoagarohexaose[J]. Int J Mol Sci, 2015, 16(3):5590-5603.
doi: 10.3390/ijms16035590 URL |
| [13] |
Chen XL, Hou YP, Jin M, et al. Expression and characterization of a novel thermostable and pH-stable β-agarase from deep-sea bacterium Flammeovirga sp. OC4[J]. J Agric Food Chem, 2016, 64(38):7251-7258.
doi: 10.1021/acs.jafc.6b02998 URL |
| [14] |
Park DY, Chi WJ, Park JS, et al. Cloning, expression, and biochemical characterization of a GH16 β-agarase AgaH71 from Pseudoalteromonas hodoensis H7[J]. Appl Biochem Biotechnol, 2015, 175(2):733-747.
doi: 10.1007/s12010-014-1294-3 URL |
| [15] |
Kim JH, Yun EJ, Seo N, et al. Enzymatic liquefaction of agarose above the Sol-gel transition temperature using a thermostable endo-type β-agarase, Aga16B[J]. Appl Microbiol Biotechnol, 2017, 101(3):1111-1120.
doi: 10.1007/s00253-016-7831-y pmid: 27664160 |
| [16] |
Krogh A, Larsson B, von Heijne G, et al. Predicting transmembrane protein topology with a hidden Markov model:application to complete genomes[J]. J Mol Biol, 2001, 305(3):567-580.
doi: 10.1006/jmbi.2000.4315 pmid: 11152613 |
| [17] |
Petersen TN, Brunak S, von Heijne G, et al. SignalP 4. 0:discriminating signal peptides from transmembrane regions[J]. Nat Methods, 2011, 8(10):785-786.
doi: 10.1038/nmeth.1701 pmid: 21959131 |
| [18] |
Chou KC, Shen HB. Cell-PLoc:a package of Web servers for predicting subcellular localization of proteins in various organisms[J]. Nat Protoc, 2008, 3(2):153-162.
doi: 10.1038/nprot.2007.494 URL |
| [19] | Marchler-Bauer A, Derbyshire MK, Gonzales NR, et al. CDD:NCBI’s conserved domain database[J]. Nucleic Acids Res, 2015, 43(Database issue):D222-D226. |
| [20] |
Song T, Xu H, Wei CC, et al. Horizontal transfer of a novel soil agarase gene from marine bacteria to soil bacteria via human microbiota[J]. Sci Rep, 2016, 6:34103.
doi: 10.1038/srep34103 pmid: 27756908 |
| [21] | 许振兴. 四株海洋新菌的鉴定及菌株HQM9T和Q1T的琼胶降解酶研究[D]. 济南: 山东大学, 2017. |
| Xu ZX. Polyphasic taxonomy analysis of four novel marine bacterial strains and studies on agar-degrading eneymes produced by strains HQM9T and Q1T[D]. Jinan: Shandong University, 2017. | |
| [22] |
Lee Y, Oh C, de Zoysa M, et al. Molecular cloning, overexpression, and enzymatic characterization of glycosyl hydrolase family 16 β-Agarase from marine bacterium Saccharophagus sp. AG21 in Escherichia coli[J]. J Microbiol Biotechnol, 2013, 23(7):913-922.
doi: 10.4014/jmb.1209.09009 URL |
| [23] |
Lee DG, Jeon MJ, Lee SH. Cloning, expression, and characterization of a glycoside hydrolase family 118 beta-agarase from Agarivorans sp. JA-1[J]. J Microbiol Biotechnol, 2012, 22(12):1692-1697.
doi: 10.4014/jmb.1209.09033 URL |
| [24] | 金佳, 江承程, 毛相朝. α-琼胶酶OUC-GaJJ96的异源表达及酶学性质[J]. 食品科学技术学报, 2020, 38(6):47-54. |
|
Jin J, Jiang CC, Mao XZ. Heterologous expression and enzymatic properties of α-agarase OUC-GaJJ96[J]. J Food Sci Technol, 2020, 38(6):47-54.
doi: 10.1046/j.1365-2621.2003.00630.x URL |
|
| [25] |
Cui X, Jiang YC, Chang LY, et al. Heterologous expression of an agarase gene in Bacillus subtilis, and characterization of the agarase[J]. Int J Biol Macromol, 2018, 120(Pt A):657-664.
doi: 10.1016/j.ijbiomac.2018.07.118 URL |
| [26] |
Kim HT, Lee S, Lee D, et al. Overexpression and molecular characterization of Aga50D from Saccharophagus degradans 2-40:an exo-type beta-agarase producing neoagarobiose[J]. Appl Microbiol Biotechnol, 2010, 86(1):227-234.
doi: 10.1007/s00253-009-2256-5 URL |
| [27] |
Liu J, Liu Z, Jiang CC, et al. Biochemical characterization and substrate degradation mode of a novel α-agarase from Catenovulum agarivorans[J]. J Agric Food Chem, 2019, 67(37):10373-10379.
doi: 10.1021/acs.jafc.9b03073 URL |
| [28] |
Jung S, Jeong BC, Hong SK, et al. Cloning, expression, and biochemical characterization of a novel acidic GH16 β-agarase, AgaJ11, from Gayadomonas joobiniege G7[J]. Appl Biochem Biotechnol, 2017, 181(3):961-971.
doi: 10.1007/s12010-016-2262-x URL |
| [29] | Aziz GM, Ali HM. Purification and characterization of agarase from Bacillus sp. H12[J]. Curr Res J Biol Sci, 2013, 5(1):13-18. |
| [30] |
Lee CH, Lee CR, Hong SK. Biochemical characterization of a novel cold-adapted agarotetraose-producing α-agarase, AgaWS5, from Catenovulum sediminis WS1-A[J]. Appl Microbiol Biotechnol, 2019, 103(20):8403-8411.
doi: 10.1007/s00253-019-10056-1 URL |
| [31] |
Fu XT, Lin H, Kim SM. Purification and characterization of a novel β-agarase, AgaA34, from Agarivorans albus YKW-34[J]. Appl Microbiol Biotechnol, 2008, 78(2):265-273.
doi: 10.1007/s00253-007-1303-3 URL |
| [32] |
Hou YP, Chen XL, Chan ZH, et al. Expression and characterization of a thermostable and pH-stable β-agarase encoded by a new gene from Flammeovirga pacifica WPAGA1[J]. Process Biochem, 2015, 50(7):1068-1075.
doi: 10.1016/j.procbio.2015.04.005 URL |
| [1] | 王帅, 冯宇梅, 白苗, 杜维俊, 岳爱琴. 大豆GmHMGR基因响应外源激素及非生物胁迫功能研究[J]. 生物技术通报, 2023, 39(7): 131-142. |
| [2] | 赵赛赛, 张小丹, 贾晓妍, 陶大炜, 刘可玉, 宁喜斌. 高产硝酸盐还原酶Staphylococcus simulans ZSJ6的复合诱变选育及其酶学性质研究[J]. 生物技术通报, 2023, 39(4): 103-113. |
| [3] | 马玉倩, 孙东辉, 岳浩峰, 辛佳瑜, 刘宁, 曹志艳. 具有辅助降解纤维素功能的大斑刚毛座腔菌糖苷水解酶GH61的鉴定、异源表达及功能分析[J]. 生物技术通报, 2023, 39(4): 124-135. |
| [4] | 陈楠楠, 王春来, 蒋振忠, 焦鹏, 关淑艳, 马义勇. 玉米ZmDHN15基因在烟草中的遗传转化及抗冷性分析[J]. 生物技术通报, 2023, 39(4): 259-267. |
| [5] | 杨俊钊, 张新蕊, 赵国柱, 郑菲. 新型GH5家族多结构域纤维素酶的结构与功能研究[J]. 生物技术通报, 2023, 39(4): 71-80. |
| [6] | 王雨辰, 丁尊丹, 关菲菲, 田健, 刘国安, 伍宁丰. 耐热漆酶ba4基因鉴定与酶学性质分析[J]. 生物技术通报, 2022, 38(8): 252-260. |
| [7] | 牛馨, 张莹, 王茂军, 刘文龙, 路福平, 李玉. 解淀粉芽胞杆菌不同整合位点对外源碱性蛋白酶表达的影响[J]. 生物技术通报, 2022, 38(4): 253-260. |
| [8] | 毛国涛, 王杰, 王凯, 王方园, 曹乐言, 张宏森, 宋安东. 水生栖热菌漆酶TaLac的性质分析及对孔雀石绿染料的脱除[J]. 生物技术通报, 2022, 38(4): 261-268. |
| [9] | 王玥, 高庆华, 董聪, 罗同阳, 王庆庆. 密码子优化的吡喃糖氧化酶基因在毕赤酵母中的表达[J]. 生物技术通报, 2022, 38(4): 269-277. |
| [10] | 常晴, 束月蓉, 王文韬, 蒋昊, 延泉德, 钱政, 高雪纯, 吴金鸿, 张勇. 来自Yeosuana marina sp. JLT21内切型海藻酸裂解酶的异源表达及酶学表征[J]. 生物技术通报, 2022, 38(2): 123-131. |
| [11] | 王博雅, 姜勇, 黄艳, 曹颖, 胡尚连. 慈竹纤维素合酶BeCesA4的克隆及功能分析[J]. 生物技术通报, 2022, 38(11): 185-193. |
| [12] | 张彤彤, 郑登俞, 吴忠义, 张中保, 于荣. 玉米NF-Y转录因子基因ZmNF-YB13响应干旱和盐胁迫的功能分析[J]. 生物技术通报, 2022, 38(10): 115-123. |
| [13] | 岑潇龙, 雷曦, 马诗云, 陈倩茹, 黄遵锡, 周峻沛, 张蕊. 金黄色葡萄球菌透明质酸裂解酶HylS的异源表达与特性研究[J]. 生物技术通报, 2022, 38(1): 157-167. |
| [14] | 田嘉慧, 封佳丽, 卢俊桦, 毛林静, 胡著然, 王莹, 楚杰. 一色齿毛菌漆酶LacT-1的分离纯化与性质研究[J]. 生物技术通报, 2021, 37(8): 186-194. |
| [15] | 廖兆民, 蔡俊, 林建国, 杜馨, 王常高. 黑曲霉葡萄糖氧化酶基因在毕赤酵母中的表达及产酶条件的优化[J]. 生物技术通报, 2021, 37(6): 97-107. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||