








Biotechnology Bulletin ›› 2025, Vol. 41 ›› Issue (12): 74-81.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0718
Previous Articles Next Articles
MAO Li-jing1,2( ), JIN Xiao-xuan3(
), JIN Xiao-xuan3( ), SHI Wan-ting3, HU Fei-yang3, ZHANG Yuan-rong1, XIONG Liang-bin2,3(
), SHI Wan-ting3, HU Fei-yang3, ZHANG Yuan-rong1, XIONG Liang-bin2,3( ), REN Lu1(
), REN Lu1( )
)
Received:2025-07-04
Online:2025-12-26
Published:2026-01-06
Contact:
XIONG Liang-bin, REN Lu
E-mail:gtmastjk@163.com;xionglb@sumhs.edu.cn;renl@sumhs.edu.cn
MAO Li-jing, JIN Xiao-xuan, SHI Wan-ting, HU Fei-yang, ZHANG Yuan-rong, XIONG Liang-bin, REN Lu. Modifying the Probiotic Escherichia coli Nissle 1917 for the Biosynthesis of Indirubin via Metabolic Engineering[J]. Biotechnology Bulletin, 2025, 41(12): 74-81.
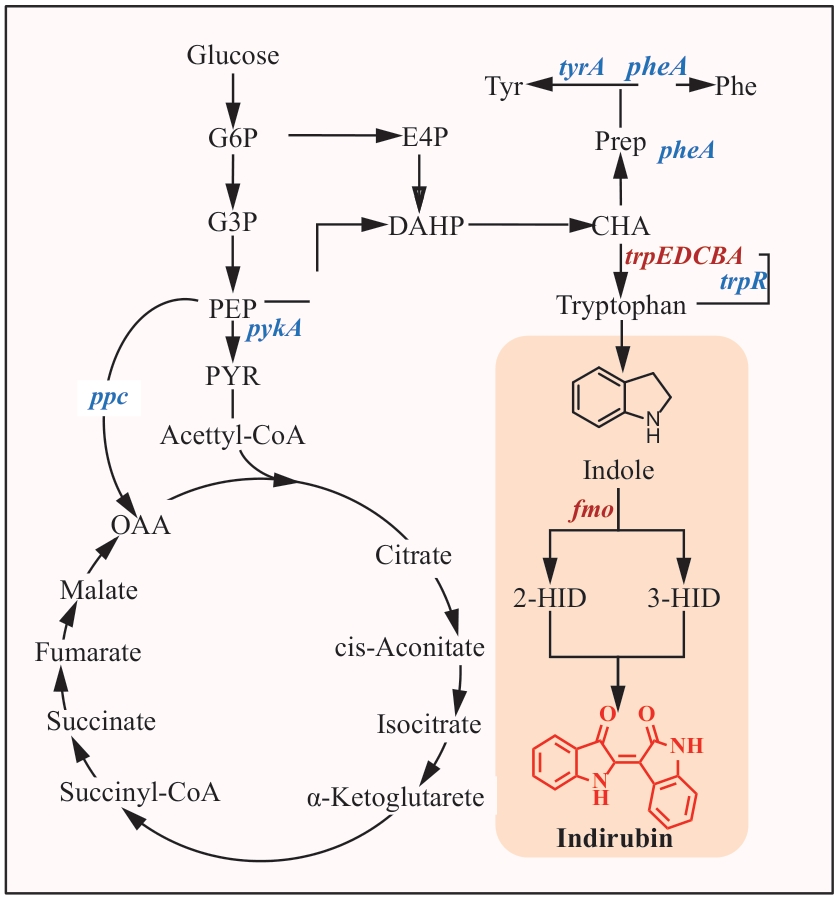
Fig. 1 Construction of the biosynthesis pathway of indirubin in Escherichia coliG6P: glucose-6-phosphate; G3P: glyceraldehyde-3-phosphate; PEP: phosphoenolpyruvate; E4P: erythrose-4-phosphate; DAHP: 3-deoxy-D-arabino-heptulosonate-7-phosphate; CHA: chorismate; Prep: prephenate; Phe: phenylalanine; Tyr: tyrosine; PYR: pyruvate; OAA: oxaloacetate; 2-HID: 2-hydroxyindole; 3-HID: 3-hydroxyindole
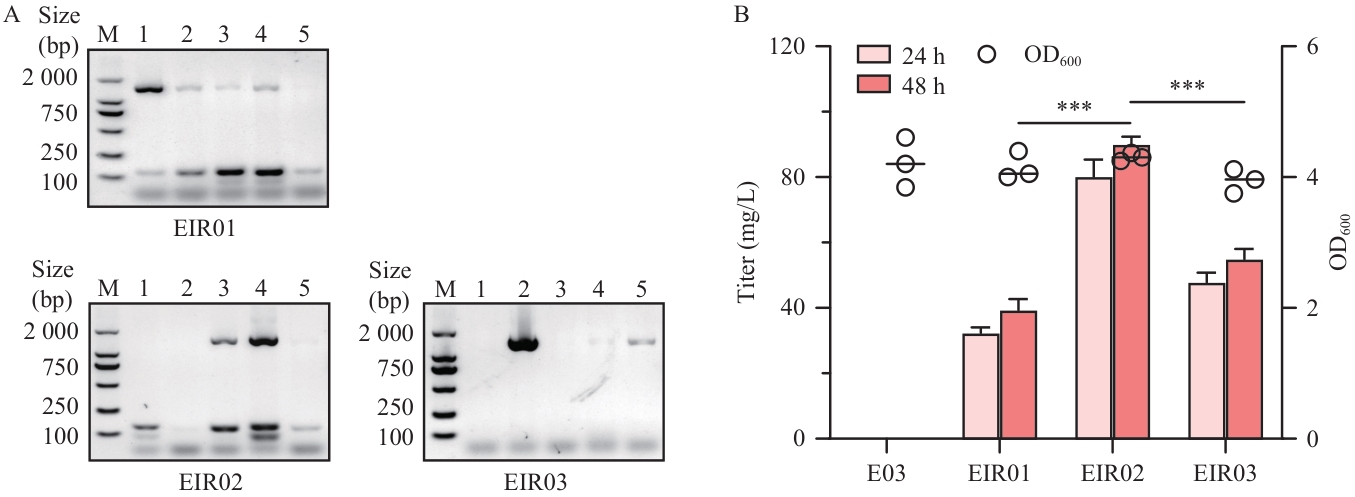
Fig. 2 Effect of introducing the fmo gene and its mutants into E. coli Nissle 1917 on the production of indirubinA: Agarose gel electrophoresis analysis of colony PCR for recombinant strains EIR01-03. Lanes show verification results for colonies of constructs: pI2e::fmo, pI2e::fmoK223R and pI2e::fmoK223R/D317S. B: Indirubin production by engineered strains EIR01-03 overexpressing fmoand its mutants. Significant differences were determined using student’s t-test (***P<0.001)
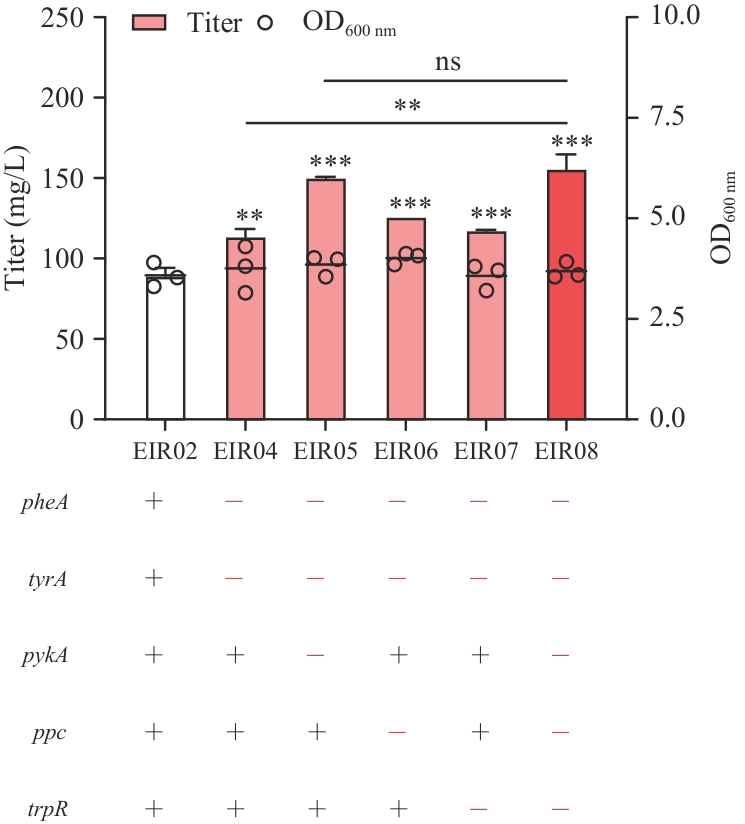
Fig. 3 Effects of knocking-out of gene related to the precursor synthesis on indirubin productionSignificant differences were determined using student’s t-test (**P<0.01; ***P<0.001; ns: not significant)
| [1] | Meijer L, Skaltsounis AL, Magiatis P, et al. GSK-3-selective inhibitors derived from Tyrian purple indirubins [J]. Chem Biol, 2003, 10(12): 1255-1266. |
| [2] | Hoessel R, Leclerc S, Endicott JA, et al. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases [J]. Nat Cell Biol, 1999, 1(1): 60-67. |
| [3] | Leclerc S, Garnier M, Hoessel R, et al. Indirubins inhibit glycogen synthase kinase-3β and CDK5/P25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer's disease [J]. J Biol Chem, 2001, 276(1): 251-260. |
| [4] | Lee JY, Shin YS, Shin HJ, et al. Production of natural indirubin from indican using non-recombinant Escherichia coli [J]. Bioresour Technol, 2011, 102(19): 9193-9198. |
| [5] | Han GH, Gim GH, Kim W, et al. Enhanced indirubin production in recombinant Escherichia coli harboring a flavin-containing monooxygenase gene by cysteine supplementation [J]. J Biotechnol, 2013, 164(2): 179-187. |
| [6] | Shi YS, Wang D, Li RS, et al. Engineering yeast subcellular compartments for increased production of the lipophilic natural products ginsenosides [J]. Metab Eng, 2021, 67: 104-111. |
| [7] | Hu ZF, Gu AD, Liang L, et al. Construction and optimization of microbial cell factories for sustainable production of bioactive dammarenediol-II glucosides [J]. Green Chem, 2019, 21(12): 3286-3299. |
| [8] | Wang WJ, Wu Y, Xu HH, et al. Accumulation mechanism of indigo and indirubin in Polygonum tinctorium revealed by metabolite and transcriptome analysis [J]. Ind Crops Prod, 2019, 141: 111783. |
| [9] | 熊亮斌, 唐红菊, 宋新巍, 等. 紫杉醇类抗肿瘤原料药生产的研究进展 [J]. 中草药, 2020, 51(15): 4042-4049. |
| Xiong LB, Tang HJ, Song XW, et al. Recent advances in synthesis of paclitaxel antitumor pharmaceutical raw materials [J]. Chin Tradit Herb Drugs, 2020, 51(15): 4042-4049. | |
| [10] | Liang FY, Xie YM, Zhang C, et al. Elucidation of the final steps in Taxol biosynthesis and its biotechnological production [J]. Nat Synth, 2025: 1-11. |
| [11] | Zhang J, Hansen LG, Gudich O, et al. A microbial supply chain for production of the anti-cancer drug vinblastine [J]. Nature, 2022, 609(7926): 341-347. |
| [12] | Hu TY, Zhou JW, Tong YR, et al. Engineering chimeric diterpene synthases and isoprenoid biosynthetic pathways enables high-level production of miltiradiene in yeast [J]. Metab Eng, 2020, 60: 87-96. |
| [13] | Liu XN, Cheng J, Zhang GH, et al. Engineering yeast for the production of breviscapine by genomic analysis and synthetic biology approaches [J]. Nat Commun, 2018, 9: 448. |
| [14] | Wang YN, Liu XN, Chen BH, et al. Metabolic engineering of Yarrowia lipolytica for scutellarin production [J]. Synth Syst Biotechnol, 2022, 7(3): 958-964. |
| [15] | Mindt M, Ferrer L, Bosch D, et al. De novo tryptophanase-based indole production by metabolically engineered Corynebacterium glutamicum [J]. Appl Microbiol Biotechnol, 2023, 107(5): 1621-1634. |
| [16] | Yang WH, Zhou JL, Gu QY, et al. Combinatorial enzymatic catalysis for bioproduction of ginsenoside compound K [J]. J Agric Food Chem, 2023, 71(7): 3385-3397. |
| [17] | Wang D, Wang JH, Shi YS, et al. Elucidation of the complete biosynthetic pathway of the main triterpene glycosylation products of Panax notoginseng using a synthetic biology platform [J]. Metab Eng, 2020, 61: 131-140. |
| [18] | Yu Z, Wei XJ, Liu LT, et al. Indirubin-3'-monoxime acts as proteasome inhibitor: Therapeutic application in multiple myeloma [J]. eBioMedicine, 2022, 78: 103950. |
| [19] | Zdioruk M, Jimenez-Macias JL, Nowicki MO, et al. PPRX-1701, a nanoparticle formulation of 6'-bromoindirubin acetoxime, improves delivery and shows efficacy in preclinical GBM models [J]. Cell Rep Med, 2023, 4(5): 101019. |
| [20] | Gowda SV, Kim NY, Harsha KB, et al. A new 1, 2, 3-triazole-indirubin hybrid suppresses tumor growth and pulmonary metastasis by mitigating the HGF/c-MET axis in hepatocellular carcinoma [J]. J Adv Res, 2025, 73: 341-356. |
| [21] | Liu XN, Zhu XX, Wang H, et al. Discovery and modification of cytochrome P450 for plant natural products biosynthesis [J]. Synth Syst Biotechnol, 2020, 5(3): 187-199. |
| [22] | Jiang Y, Chen B, Duan CL, et al. Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system [J]. Appl Environ Microbiol, 2015, 81(7): 2506-2514. |
| [23] | Yin HF, Chen HP, Yan M, et al. Efficient bioproduction of indigo and indirubin by optimizing a novel terpenoid cyclase XiaI in Escherichia coli [J]. ACS Omega, 2021, 6(31): 20569-20576. |
| [24] | Sun BY, Sui HL, Liu ZW, et al. Structure-guided engineering of a flavin-containing monooxygenase for the efficient production of indirubin [J]. Bioresour Bioprocess, 2022, 9(1): 70. |
| [25] | Du JK, Li YH, Chen ZZ, et al. Functional characterization of a novel flavin reductase from a deep-sea sediment metagenomic library and its application for indirubin production [J]. Appl Environ Microbiol, 2024, 90(6): e00429-24. |
| [26] | Dong MM, Song L, Xu JQ, et al. Improved cryptic plasmids in probiotic Escherichia coli Nissle 1917 for antibiotic-free pathway engineering [J]. Appl Microbiol Biotechnol, 2023, 107(16): 5257-5267. |
| [27] | Dong MM, Li YX, Xu M, et al. An Escherichia coli Nissle 1917-based live therapeutics platform with integrated phage resistance and programmable temperature sensitivity [J]. J Control Release, 2025, 387: 114188. |
| [28] | 陈博, 宋凯, 何亚文. 微生物中邻氨基苯甲酸代谢与功能研究进展 [J]. 微生物前沿, 2024, 13(3): 175-186. |
| Chen B, Song K, He YW. The metabolism and functions of anthranilic acid in microorganisms [J]. Adv Microbiol, 2024, 13(3): 175-186. | |
| [29] | Rothman SC, Voorhies M, Kirsch JF. Directed evolution relieves product inhibition and confers in vivo function to a rationally designed tyrosine aminotransferase [J]. Protein Sci, 2004, 13(3): 763-772. |
| [30] | Du LH, Zhang Z, Xu QY, et al. Central metabolic pathway modification to improve L-tryptophan production in Escherichia coli [J]. Bioengineered, 2019, 10(1): 59-70. |
| [31] | Noda S, Shirai T, Oyama S, et al. Metabolic design of a platform Escherichia coli strain producing various chorismate derivatives [J]. Metab Eng, 2016, 33: 119-129. |
| [32] | 翁可欣, 张明亮, 李力. 微生物合成5-羟基色氨酸的研究进展 [J]. 热带生物学报, 2023, 14(1): 42-49. |
| Weng KX, Zhang ML, Li L. Advances in microbial synthesis of 5-hydroxytryptophan [J]. J Trop Biol, 2023, 14(1): 42-49. |
| [1] | LIU Yu-shi, LI Zhen, ZOU Yu-chen, TANG Wei-wei, LI Bin. Advances in Spatial Metabolomics in Medicinal Plants [J]. Biotechnology Bulletin, 2025, 41(9): 22-31. |
| [2] | WANG Bin, LIN Chong, YUAN Xiao, JIANG Yuan-yuan, WANG Yu-kun, XIAO Yan-hui. Cloning of bHLH Transcription Factor UNE10 and Its Regulatory Roles in the Biosynthesis of Volatile Compounds in Clove Basil [J]. Biotechnology Bulletin, 2025, 41(9): 207-218. |
| [3] | CAI Ru-feng, YANG Yu-xuan, YU Ji-zheng, LI Jia-nan. Artificial Intelligence Transforms Protein Engineering: From Structural Analysis to Synthetic Biology through Algorithmic Advancements [J]. Biotechnology Bulletin, 2025, 41(8): 1-10. |
| [4] | HUANG Xu-sheng, ZHOU Ya-li, CHAI Xu-dong, WEN Jing, WANG Ji-ping, JIA Xiao-yun, LI Run-zhi. Cloning of Plastidial PfLPAT1B Gene from Perilla frutescens and Its Functional Analysis in Oil Biosynthesis [J]. Biotechnology Bulletin, 2025, 41(7): 226-236. |
| [5] | GAO Jing, CHENG Yi-cun, GAO Ming, ZHAO Yun-xiao, WANG Yang-dong. Regulation of Plant Tannin Synthesis and Mechanisms of Its Responses to Environment [J]. Biotechnology Bulletin, 2025, 41(7): 49-59. |
| [6] | WU Ya, YAO Run, YANG Han-ting, LIU Wei, YANG Shuai, SONG Chi, CHEN Shi-lin. Genome-wide Identification and Expression Analysis of SDR Gene Family in Mentha suaveolens ‘Variegata’ [J]. Biotechnology Bulletin, 2025, 41(5): 175-185. |
| [7] | LU Tian-yi, LI Ai-peng, FEI Qiang. Research Progress in the Biosynthesis of Polylactic Acid [J]. Biotechnology Bulletin, 2025, 41(4): 47-60. |
| [8] | LI Xiao-ming, SHANG Xiu-hua, WANG You-shuang, WU Zhi-hua. Research Progress in Benzoxazinoids in Plants [J]. Biotechnology Bulletin, 2025, 41(4): 9-20. |
| [9] | WANG Jia, GAO Ming, ZHAO Yun-xiao, CHEN Yi-cun, WANG Yang-dong. Cytochrome P450 Involved in Secondary Metabolites Biosynthesis in Response to Biotic Stresses [J]. Biotechnology Bulletin, 2025, 41(12): 27-39. |
| [10] | ZHOU Si-yan, DING Wei-quan, DONG Ping, WENG Han-zhi, XU Rui-rui, KANG Zhen. Advances in Synthetic Biology Platform Development and Application for Escherichiacoli Nissle 1917 [J]. Biotechnology Bulletin, 2025, 41(11): 47-61. |
| [11] | XU Xin-xin, LI Yan-jun, ZHANG Wei, HUANG Huo-qing, LUO Hui-ying, YAO Bin. Artificial Starch Biosynthesis Technology: Progress, Challenges, and Prospects [J]. Biotechnology Bulletin, 2025, 41(11): 22-27. |
| [12] | LYU Huan-huan, ZHANG Gao-yang, WANG Sai-di, SUN Zhong-ke, LI Cheng-wei, LUO De-ping. Advances in the Biosynthesis of 5-aminolevulinic Acid (5-ALA) [J]. Biotechnology Bulletin, 2025, 41(11): 75-88. |
| [13] | JI Meng-ran, ZHANG Rui-ying, LIU Hong-dan, FENG Wei-meng, LIU Xiu-yu, MA Rui, CHEN Sui-qing. Combined Metabolome and Transcriptome Analysis of the Differences in Terpenoids between New and Old Leaves of Artemisia argyi H. Lév. & Vaniot [J]. Biotechnology Bulletin, 2025, 41(10): 277-291. |
| [14] | NIE Zhu-xin, GUO Jin, QIAO Zi-yang, LI Wei-wei, ZHANG Xue-yan, LIU Chun-yang, WANG Jing. Transcriptome Analysis of the Anthocyanin Biosynthesis in the Fruit Development Processes of Lycium ruthenicum Murr. [J]. Biotechnology Bulletin, 2024, 40(8): 106-117. |
| [15] | MA Xiao-xiang, MA Ze-yuan, LIU Ya-yue, ZHOU Long-jian, HE Yi-fan, ZHANG Yi. Effects of Simulated Mutational Biosynthetic Regulation on the Secondary Metabolites of Aspergillus terreus C23-3 [J]. Biotechnology Bulletin, 2024, 40(8): 275-287. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||