








Biotechnology Bulletin ›› 2024, Vol. 40 ›› Issue (4): 23-32.doi: 10.13560/j.cnki.biotech.bull.1985.2023-1084
Previous Articles Next Articles
HUA Zi-qing1,2( ), ZHOU Jing-yuan2,3, DONG He-zhong1,2(
), ZHOU Jing-yuan2,3, DONG He-zhong1,2( )
)
Received:2023-11-20
Online:2024-04-26
Published:2024-04-30
Contact:
DONG He-zhong
E-mail:huazq@163.com;donghezhong@163.com
HUA Zi-qing, ZHOU Jing-yuan, DONG He-zhong. Development of Hypocotyls and Apical Hooks in Dicotyledons and Their Regulatory Mechanisms for Seedling Emergence[J]. Biotechnology Bulletin, 2024, 40(4): 23-32.
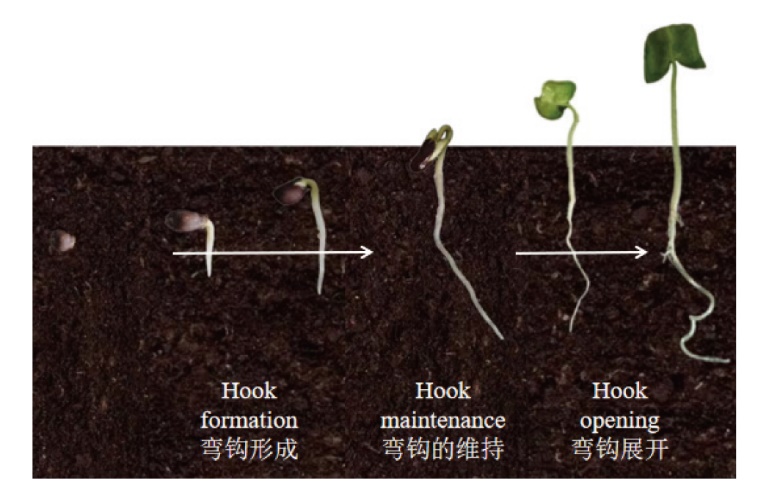
Fig. 1 Process of seed germination and seedling emergence in dicotyledonous plants(cotton as an example) During the early stage of seedling emergence, cells on both sides of the apical hypocotyl grow differentially to form the apical hook. Under the mechanical pressure of the soil, the differential growth of cells on the inner and outer sides of the apical hook is intensified to entere the maintenance stage, while the hypocotyl thickened laterally. With the upward growth of the seedling, the mechanical pressure of the soil decreases, the hypocotyl elongates rapidly, the growth rate of the inner cells of the apical hook increases, and finally the apical hook and the cotyledons get unfolded to complete the successful emergence
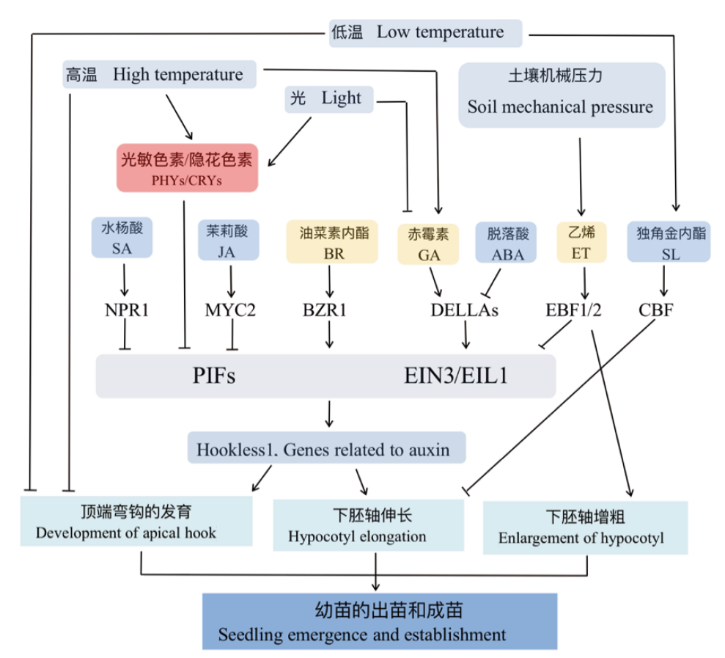
Fig. 2 Factors influencing the development of apical hook and hypocotyl and their mechanisms in regulating seedling emergence and stand establishment Environmental signals, such as light and temperature, interact with phytohormones like ethylene and gibberellin, to jointly regulate the expressions of genes related to the development of hypocotyls and apical hooks, and to promote seedling emergence and stand establishment. Normal arrows indicate positive regulatory effects, while T-arrows indicate negative regulatory effects. PHYs/CRYs: Phytochromes/Cryptochromes; SA: salicylic acid; JA: jasmonic acid; GA: gibberellic acid; ABA: abscisic acid; BR: brassinosteroids; ET: ethylene; SL: strigolactone; NPR1: salicylic acid receptor protein; MYC2: jasmonic acid signaling pathway transcription factors; DELLAs: DELLA family proteins; BZR1: brassinosteroids receptor protein; EBF1/2: ethylene insensitive 3 binding F-box protein 1/2; CBF: AP2/EREBP transcription factor family members
| [1] | 于延文. 乙烯调控拟南芥HY5蛋白稳定性和幼苗下胚轴生长[D]. 北京: 中国农业科学院, 2014. |
| Yu YW. Ethylene regulates Arabidopsis HY5 protein stability and seedling hypocotyl growth[D]. Beijing: Chinese Academy of Agricultural Sciences, 2014. | |
| [2] | 岳剑茹, 赫云建, 邱天麒, 等. 植物微管骨架参与下胚轴伸长调节机制研究进展[J]. 植物学报, 2021, 56(3): 363-371. |
| Yue JR, He YJ, Qiu TQ, et al. Research advances in the molecular mechanisms of plant microtubules in regulating hypocotyl elongation[J]. Chin Bull Bot, 2021, 56(3): 363-371. | |
| [3] |
Raz V, Ecker JR. Regulation of differential growth in the apical hook of Arabidopsis[J]. Development, 1999, 126(16): 3661-3668.
doi: 10.1242/dev.126.16.3661 pmid: 10409511 |
| [4] | Josse EM, Halliday KJ. Skotomorphogenesis: the dark side of light signalling[J]. Curr Biol, 2008, 18(24): R1144-R1146. |
| [5] | 杨东旭, 王昕源, 黄迪, 等. 高等植物下胚轴生长发育研究进展[J]. 黑龙江农业科学, 2023(1): 118-123. |
| Yang DX, Wang XY, Huang D, et al. Advances of research on hypocotyl growth in higher plants[J]. Heilongjiang Agric Sci, 2023(1): 118-123. | |
| [6] | 朱蠡庆, 王伯初, 付雪, 等. 膨压在植物细胞生长中的作用[J]. 生物物理学报, 2013, 29(8): 583-593. |
| Zhu LQ, Wang BC, Fu X, et al. Turgor pressure in plant cell growth[J]. Acta Biophys Sin, 2013, 29(8): 583-593. | |
| [7] |
Hu HZ, Zhang R, Feng SQ, et al. Three AtCesA6-like members enhance biomass production by distinctively promoting cell growth in Arabidopsis[J]. Plant Biotechnol J, 2018, 16(5): 976-988.
doi: 10.1111/pbi.2018.16.issue-5 URL |
| [8] |
Zhong SW, Shi H, Xue C, et al. A molecular framework of light-controlled phytohormone action in Arabidopsis[J]. Curr Biol, 2012, 22(16): 1530-1535.
doi: 10.1016/j.cub.2012.06.039 URL |
| [9] | Sun JB, Ma QQ, Mao TL. Ethylene regulates the Arabidopsis microtubule-associated protein WAVE-DAMPENED2-LIKE5 in etiolated hypocotyl elongation[J]. Plant Physiol, 2015, 169(1): 325-337. |
| [10] |
Jing YJ, Lin RC. Transcriptional regulatory network of the light signaling pathways[J]. New Phytol, 2020, 227(3): 683-697.
doi: 10.1111/nph.16602 pmid: 32289880 |
| [11] | 江薇, 肖宁, 陆怡, 等. 植物光敏色素作用因子PIFs的生物学功能[J]. 植物生理学报, 2014, 50(6): 698-706. |
|
Jiang W, Xiao N, Lu Y, et al. Biological function of phytochrome-interacting factors in plant[J]. Plant Physiol J, 2014, 50(6): 698-706.
doi: 10.1104/pp.50.6.698 URL |
|
| [12] | Lin CT. Blue light receptors and signal transduction[J]. Plant Cell, 2002, 14(Suppl): S207-S225. |
| [13] | 唐冬英, 赵小英, 谢敏敏, 等. 隐花素在生长素调控拟南芥下胚轴伸长中的作用分析[J]. 生命科学研究, 2014, 18(2): 124-128, 183. |
| Tang DY, Zhao XY, Xie MM, et al. Effect of cryptochrome on IAA regulating hypocotyls elongation in Arabidopsis thaliana[J]. Life Sci Res, 2014, 18(2): 124-128, 183. | |
| [14] | Xiong HB, Lu DD, Li ZY, et al. The DELLA-ABI4-HY5 module integrates light and gibberellin signals to regulate hypocotyl elongation[J]. Plant Commun, 2023, 4(5): 100597. |
| [15] |
Han X, Yu H, Yuan RR, et al. Arabidopsis transcription factor TCP5 controls plant thermomorphogenesis by positively regulating PIF4 activity[J]. iScience, 2019, 15: 611-622.
doi: 10.1016/j.isci.2019.04.005 URL |
| [16] | Kim S, Hwang G, Kim S, et al. The epidermis coordinates thermoresponsive growth through the phyB-PIF4-auxin pathway[J]. Nat Commun, 2020, 11(1): 1053. |
| [17] |
Claeys H, De Bodt S, Inzé D. Gibberellins and DELLAs: central nodes in growth regulatory networks[J]. Trends Plant Sci, 2014, 19(4): 231-239.
doi: 10.1016/j.tplants.2013.10.001 pmid: 24182663 |
| [18] | Wang X, Li ZY, Shi YT, et al. Strigolactones promote plant freezing tolerance by releasing the WRKY41-mediated inhibition of CBF/DREB1 expression[J]. EMBO J, 2023, 42(19): e112999. |
| [19] |
Wang L, Xu Q, Yu H, et al. Strigolactone and karrikin signaling pathways elicit ubiquitination and proteolysis of SMXL2 to regulate hypocotyl elongation in Arabidopsis[J]. Plant Cell, 2020, 32(7): 2251-2270.
doi: 10.1105/tpc.20.00140 URL |
| [20] |
Oh E, Zhu JY, Bai MY, et al. Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl[J]. eLife, 2014, 3: e03031.
doi: 10.7554/eLife.03031 URL |
| [21] | Yu ZP, Ma JX, Zhang MY, et al. Auxin promotes hypocotyl elongation by enhancing BZR1 nuclear accumulation in Arabidopsis[J]. Sci Adv, 2023, 9(1): eade2493. |
| [22] |
Feng SH, Martinez C, Gusmaroli G, et al. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins[J]. Nature, 2008, 451(7177): 475-479.
doi: 10.1038/nature06448 |
| [23] |
Zheng YY, Cui XF, Su L, et al. Jasmonate inhibits COP1 activity to suppress hypocotyl elongation and promote cotyledon opening in etiolated Arabidopsis seedlings[J]. Plant J, 2017, 90(6): 1144-1155.
doi: 10.1111/tpj.2017.90.issue-6 URL |
| [24] | 马海燕. 葡萄生长过程中内源激素含量变化的研究[D]. 杨凌: 西北农林科技大学, 2007. |
| Ma HY. Changes of endogenous hormones in grapevine during its development[D]. Yangling: Northwest A & F University, 2007. | |
| [25] | Lorrai R, Boccaccini A, Ruta V, et al. Abscisic acid inhibits hypocotyl elongation acting on gibberellins, DELLA proteins and auxin[J]. AoB Plants, 2018, 10(5): ply061. |
| [26] | 王红飞, 李福凯, 尚庆茂. 温度、光照强度及渗透势对普通白菜下胚轴伸长的交互作用[J]. 中国蔬菜, 2017(11): 46-52. |
| Wang HF, Li FK, Shang QM. Interaction of temperature, light intensity and osmotic potential on Brassica campestris L. ssp. chinensis(L.) makino var. communis tsen et lee hypocotyl elongation[J]. China Veg, 2017(11): 46-52. | |
| [27] | 宋雨函, 张锐. 高等植物下胚轴伸长的调控机制[J]. 生命的化学, 2021, 41(6): 1116-1125. |
| Song YH, Zhang R. Regulation mechanism of hypocotyl elongation in higher plants[J]. Chem Life, 2021, 41(6): 1116-1125. | |
| [28] |
张冬梅, 张艳军, 李存东, 等. 论棉花轻简化栽培[J]. 棉花学报, 2019, 31(2): 163-168.
doi: 10.11963/1002-7807.zdmdhz.20190313 |
|
Zhang DM, Zhang YJ, Li CD, et al. On light and simplified cotton cultivation[J]. Cotton Sci, 2019, 31(2): 163-168.
doi: 10.11963/1002-7807.zdmdhz.20190313 |
|
| [29] |
周静远, 孔祥强, 张艳军, 等. 基于种子萌发出苗过程中弯钩建成和下胚轴生长的棉花出苗壮苗机制与技术[J]. 作物学报, 2022, 48(5): 1051-1058.
doi: 10.3724/SP.J.1006.2022.14116 |
|
Zhou JY, Kong XQ, Zhang YJ, et al. Mechanism and technology of stand establishment improvements through regulating the apical hook formation and hypocotyl growth during seed germination and emergence in cotton[J]. Acta Agron Sin, 2022, 48(5): 1051-1058.
doi: 10.3724/SP.J.1006.2022.14116 URL |
|
| [30] | Shen X, Li YL, Pan Y, et al. Activation of HLS1 by mechanical stress via ethylene-stabilized EIN3 is crucial for seedling soil emergence[J]. Front Plant Sci, 2016, 7: 1571. |
| [31] | Du MM, Bou Daher F, Liu YY, et al. Biphasic control of cell expansion by auxin coordinates etiolated seedling development[J]. Sci Adv, 2022, 8(2): eabj1570. |
| [32] |
刘旦梅, 裴雁曦. 双子叶植物幼苗顶端弯钩发育的分子机制[J]. 中国生物化学与分子生物学报, 2018, 34(11): 1138-1145.
doi: 10.13865/j.cnki.cjbmb.2018.11.02 |
| Liu DM, Pei YX. Molecular mechanism of the development of dicotyledonous seedling apical hook[J]. Chin J Biochem Mol Biol, 2018, 34(11): 1138-1145. | |
| [33] |
曹文杰, 李贵生. 生长素输出载体PIN蛋白的质膜定位机制[J]. 植物学报, 2016, 51(2): 265-273.
doi: 10.11983/CBB15017 |
| Cao WJ, Li GS. Plasma membrane positioning mechanism of auxin efflux carrier PIN proteins[J]. Chin Bull Bot, 2016, 51(2): 265-273. | |
| [34] | Leyser O. Plant hormones: ins and outs of auxin transport[J]. Curr Biol, 1999, 9(1): R8-R10. |
| [35] |
Zádníková P, Petrásek J, Marhavy P, et al. Role of PIN-mediated auxin efflux in apical hook development of Arabidopsis thaliana[J]. Development, 2010, 137(4): 607-617.
doi: 10.1242/dev.041277 pmid: 20110326 |
| [36] |
Lehman A, Black R, Ecker JR. HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl[J]. Cell, 1996, 85(2): 183-194.
doi: 10.1016/s0092-8674(00)81095-8 pmid: 8612271 |
| [37] |
Peng Y, Zhang D, Qiu YP, et al. Growth asymmetry precedes differential auxin response during apical hook initiation in Arabidopsis[J]. J Integr Plant Biol, 2022, 64(1): 5-22.
doi: 10.1111/jipb.v64.1 URL |
| [38] |
Cao M, Chen R, Li P, et al. TMK1-mediated auxin signalling regulates differential growth of the apical hook[J]. Nature, 2019, 568(7751): 240-243.
doi: 10.1038/s41586-019-1069-7 |
| [39] | Ren H, Park MY, Spartz AK, et al. A subset of plasma membrane-localized PP2C.D phosphatases negatively regulate SAUR-mediated cell expansion in Arabidopsis[J]. PLoS Genet, 2018, 14(6): e1007455. |
| [40] |
Aizezi Y, Shu HZ, Zhang LL, et al. Cytokinin regulates apical hook development via the coordinated actions of EIN3/EIL1 and PIF transcription factors in Arabidopsis[J]. J Exp Bot, 2022, 73(1): 213-227.
doi: 10.1093/jxb/erab403 URL |
| [41] | Holtkotte X, Ponnu J, Ahmad M, et al. The blue light-induced interaction of cryptochrome 1 with COP1 requires SPA proteins during Arabidopsis light signaling[J]. PLoS Genet, 2017, 13(10): e1007044. |
| [42] |
Oh J, Park E, Song K, et al. PHYTOCHROME INTERACTING FACTOR8 inhibits phytochrome A-mediated far-red light responses in Arabidopsis[J]. Plant Cell, 2020, 32(1): 186-205.
doi: 10.1105/tpc.19.00515 URL |
| [43] |
Wang JJ, Sun N, Zhang FF, et al. SAUR17 and SAUR50 differentially regulate PP2C-D1 during apical hook development and Cotyledon opening in Arabidopsis[J]. Plant Cell, 2020, 32(12): 3792-3811.
doi: 10.1105/tpc.20.00283 URL |
| [44] |
Xiong JW, Yang FB, Wei F, et al. Inhibition of SIZ1-mediated SUMOylation of HOOKLESS1 promotes light-induced apical hook opening in Arabidopsis[J]. Plant Cell, 2023, 35(6): 2027-2043.
doi: 10.1093/plcell/koad072 URL |
| [45] |
Shi H, Shen X, Liu RL, et al. The red light receptor phytochrome B directly enhances substrate-E3 ligase interactions to attenuate ethylene responses[J]. Dev Cell, 2016, 39(5): 597-610.
doi: 10.1016/j.devcel.2016.10.020 pmid: 27889482 |
| [46] |
Xu P, Chen HR, Li T, et al. Blue light-dependent interactions of CRY1 with GID1 and DELLA proteins regulate gibberellin signaling and photomorphogenesis in Arabidopsis[J]. Plant Cell, 2021, 33(7): 2375-2394.
doi: 10.1093/plcell/koab124 URL |
| [47] |
Jung JH, Domijan M, Klose C, et al. Phytochromes function as thermosensors in Arabidopsis[J]. Science, 2016, 354(6314): 886-889.
doi: 10.1126/science.aaf6005 URL |
| [48] |
Jin HH, Pang L, Fang S, et al. High ambient temperature antagonizes ethylene-induced exaggerated apical hook formation in etiolated Arabidopsis seedlings[J]. Plant Cell Environ, 2018, 41(12): 2858-2868.
doi: 10.1111/pce.v41.12 URL |
| [49] |
Shibasaki K, Uemura M, Tsurumi S, et al. Auxin response in Arabidopsis under cold stress: underlying molecular mechanisms[J]. Plant Cell, 2009, 21(12): 3823-3838.
doi: 10.1105/tpc.109.069906 URL |
| [50] |
Wang YC, Guo HW. On hormonal regulation of the dynamic apical hook development[J]. New Phytol, 2019, 222(3): 1230-1234.
doi: 10.1111/nph.15626 pmid: 30537131 |
| [51] |
Guo HW, Ecker JR. Plant responses to ethylene gas are mediated by SCF(EBF1/EBF2)-dependent proteolysis of EIN3 transcription factor[J]. Cell, 2003, 115(6): 667-677.
doi: 10.1016/s0092-8674(03)00969-3 pmid: 14675532 |
| [52] |
An FY, Zhang X, Zhu ZQ, et al. Coordinated regulation of apical hook development by gibberellins and ethylene in etiolated Arabidopsis seedlings[J]. Cell Res, 2012, 22(5): 915-927.
doi: 10.1038/cr.2012.29 |
| [53] |
Wang JJ, Sun N, Zheng LD, et al. Brassinosteroids promote etiolated apical structures in darkness by amplifying the ethylene response via the EBF-EIN3/PIF3 circuit[J]. Plant Cell, 2023, 35(1): 390-408.
doi: 10.1093/plcell/koac316 URL |
| [54] |
Chory J, Nagpal P, Peto CA. Phenotypic and genetic analysis of det2, a new mutant that affects light-regulated seedling development in Arabidopsis[J]. Plant Cell, 1991, 3(5): 445-459.
doi: 10.2307/3869351 URL |
| [55] |
Huang PX, Dong Z, Guo PR, et al. Salicylic acid suppresses apical hook formation via NPR1-mediated repression of EIN3 and EIL1 in Arabidopsis[J]. Plant Cell, 2020, 32(3): 612-629.
doi: 10.1105/tpc.19.00658 URL |
| [56] |
Zhang JJ, Chen WY, Li XP, et al. Jasmonates regulate apical hook development by repressing brassinosteroid biosynthesis and signaling[J]. Plant Physiol, 2023, 193(2): 1561-1579.
doi: 10.1093/plphys/kiad399 pmid: 37467431 |
| [57] |
Mazzella MA, Casal JJ, Muschietti JP, et al. Hormonal networks involved in apical hook development in darkness and their response to light[J]. Front Plant Sci, 2014, 5: 52.
doi: 10.3389/fpls.2014.00052 pmid: 24616725 |
| [58] | 晋欢欢. 高温抑制拟南芥顶端弯钩形成与HLS1促进热形态建成的机理研究[D]. 南京: 南京师范大学, 2020. |
| Jin HH. Mechanism of high temperature inhibition of apical curvature formation in Arabidopsis thaliana and promotion of thermomorphogenesis by HLS1[D]. Nanjing: Nanjing Normal University, 2020. | |
| [59] |
You MP, Barbetti MJ. Severity of phytophthora root rot and pre-emergence damping-off in subterranean clover influenced by moisture, temperature, nutrition, soil type, cultivar and their interactions[J]. Plant Pathol, 2017, 66(7): 1162-1181.
doi: 10.1111/ppa.2017.66.issue-7 URL |
| [1] | LIN Xin-yan, ZHANG Chuan-zhong, DAI Bing, WANG Xin-heng, LIU Jian-feng, WEN Li, XU Xing-jian, FANG Jun. Advances in Genetic and Molecular Mechanisms of Pre-harvest Sprouting in Rice [J]. Biotechnology Bulletin, 2024, 40(1): 24-31. |
| [2] | WEI Xin-xin, LAN Hai-yan. Advances in the Regulation of Plant MYB Transcription Factors in Secondary Metabolism and Stress Response [J]. Biotechnology Bulletin, 2022, 38(8): 12-23. |
| [3] | SUN Man-luan, GE Sai, BU Jia, ZHU Zhuang-yan. Regulation Mechanism of Ribonucleases in Escherichia coli [J]. Biotechnology Bulletin, 2022, 38(3): 234-245. |
| [4] | ZOU Kun, LU Li-li, Collins Asiamah Amponsah, XUE Yuan, ZHANG Shao-wei, SU Ying, ZHAO Zhi-hui. Research Progress on Mechanism of Poultry Follicular Atresia [J]. Biotechnology Bulletin, 2020, 36(4): 185-191. |
| [5] | WEI Ming-ming, ZENG Xia, AN Ze-wei, HU Yan-shi, HUANG Xiao, LI Wei-guo. Advances in the Maintenance and Termination of Floral Meristem Regulated by C-type Floral Organ Gene AGAMOUS(AG) [J]. Biotechnology Bulletin, 2020, 36(1): 135-143. |
| [6] | LI Xiao-yuan, XIE Li-nan. Research Progress in Na+ Regulation Mechanism of Plants Under Salt Stress [J]. Biotechnology Bulletin, 2019, 35(7): 148-155. |
| [7] | QIANG Xiao-nan, LI Xin, CHEN Jia, LIAO Hong-dong, YU Feng. Preliminary Analysis of Functional Diversity of RALF Peptide Family in Arabidopsis thaliana [J]. Biotechnology Bulletin, 2019, 35(1): 2-10. |
| [8] | WANG Shuo, DING Lan, LIU Jian-xiang, HAN Jia-jia. PIF4-Regulated Thermo-responsive Genes in Arabidopsis [J]. Biotechnology Bulletin, 2018, 34(7): 57-65. |
| [9] | KUANG Yong-jie, LIU Lang, YAN Fang, REN Bin, YAN Da-qi, ZHANG Da-wei, LIN Hong-hui, ZHOU Huan-bin. Functions of Phytohormones During the Interactions Between Rice, Pathogens [J]. Biotechnology Bulletin, 2018, 34(2): 74-86. |
| [10] | LIU Xiao-wei, YANG Xiu-yan, LIU Zheng-xiang, WU Hai-wen, ZHANG Hua-xin, ZHU Jian-feng. Role of MicroRNA in Plant Resistance to Salt Stress [J]. Biotechnology Bulletin, 2017, 33(12): 12-21. |
| [11] | YANG Xian-you, HUANG You-jun, ZHANG Tong, HUANG Chun-ying. Advances on Transcriptional Activator AtWRI1 of Arabidopsis [J]. Biotechnology Bulletin, 2016, 32(6): 13-18. |
| [12] | WEI Ming-ming, LI Wei-guo, GAO Xin-sheng, HUANG Xiao. Research Progress of the Physiological and Molecular Regulation Mechanism of Hevea brasiliensis in Response to Ethephon Stimulation [J]. Biotechnology Bulletin, 2016, 32(3): 1-11. |
| [13] | Sun Xiaomei, Han Ningning, Yang Hongguang, Yang Panpan. cDNA-AFLP Analysis of Dormancy-related Genes in Seed Hypocotyl of Paeonia lactiflora [J]. Biotechnology Bulletin, 2015, 31(6): 116-121. |
| [14] | Jia Hongfang, Zhang Hongying, Liu Weizhi, Cui Hong, Liu Guoshun. Function and Regulation Mechanisms of Nitrate Transporters in Higher Plants [J]. Biotechnology Bulletin, 2014, 30(6): 14-21. |
| [15] | Li Qiang, Wu Jianming, Liang He, Huang Xing, Qiu Lihang. Gibberellins Biosynthesis and Signaling Transduction Pathway in Higher Plant [J]. Biotechnology Bulletin, 2014, 30(10): 16-22. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||