








生物技术通报 ›› 2023, Vol. 39 ›› Issue (3): 123-132.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0819
王海龙1( ), 李雨倩1,2, 王勃3, 邢国芳2(
), 李雨倩1,2, 王勃3, 邢国芳2( ), 张杰伟1(
), 张杰伟1( )
)
收稿日期:2022-07-02
出版日期:2023-03-26
发布日期:2023-04-10
通讯作者:
邢国芳,女,博士,副教授,研究方向:谷子分子生物学;E-mail: sxauxgf@126.com;作者简介:王海龙,男,博士,研究方向:谷子分子生物学;E-mail:whldyhm@sina.com
基金资助:
WANG Hai-long1( ), LI Yu-qian1,2, WANG Bo3, XING Guo-fang2(
), LI Yu-qian1,2, WANG Bo3, XING Guo-fang2( ), ZHANG Jie-wei1(
), ZHANG Jie-wei1( )
)
Received:2022-07-02
Published:2023-03-26
Online:2023-04-10
摘要:
丝裂原激活的蛋白激酶(MAPK)是一类丝氨酸/苏氨酸蛋白质激酶,在植物生长发育、响应逆境胁迫及激素信号转导等方面具有重要作用。以谷子品种豫谷1号为试材,克隆与拟南芥AtMAPK3同源性最高的谷子SiMAPK3基因,并系统利用生物信息学方法分析SiMAPK3蛋白理化性质、结构与功能;利用RT-qPCR技术检测SiMAPK3基因在谷子拔节前期不同组织和不同非生物逆境胁迫下的表达水平。结果表明,谷子SiMAPK3基因开放阅读框为1 128 bp,编码一个含有376个氨基酸的蛋白,预测蛋白分子量为43 427.85 Da,等电点为5.46。谷子SiMAPK3为不含信号肽的亲水性膜外蛋白,其二级结构包含44.27%的α螺旋、14.67%的β折叠、5.07%的延伸链及36.00%的无规则卷曲,其第44-328位氨基酸之间含有Pkinase保守结构域,属于MAPK蛋白激酶家族。谷子SiMAPK3三级结构与拟南芥MAPK具有很高的相似度,存在着11个丝氨酸、8个苏氨酸、4个酪氨酸及大量潜在磷酸化位点。RT-qPCR分析表明,SiMAPK3在拔节前期谷子根、茎和叶中均有表达,其在叶片中的表达量最高,约为其在根中表达量的25倍。SiMAPK3响应了低温(4℃)、高盐(300 mmol/L NaCl)、干旱和ABA(200 μmol/L)、JA(200 μmol/L)的胁迫。在低温胁迫12 h后,SiMAPK3的表达量上调了16倍,在高盐胁迫3 h后,SiMAPK3的表达量上调了8倍。谷子SiMAPK3基因的克隆及功能分析,为进一步解析谷子叶片生长发育及响应低温和高盐等胁迫信号转导过程提供重要依据。
王海龙, 李雨倩, 王勃, 邢国芳, 张杰伟. 谷子SiMAPK3基因的克隆和表达特性分析[J]. 生物技术通报, 2023, 39(3): 123-132.
WANG Hai-long, LI Yu-qian, WANG Bo, XING Guo-fang, ZHANG Jie-wei. Isolation and Expression Analysis of SiMAPK3 in Setaria italica L.[J]. Biotechnology Bulletin, 2023, 39(3): 123-132.

图1 谷子SiMAPK3的序列与结构分析 A:谷子SiMAPK3蛋白的疏水性;B:谷子SiMAPK3二级结构分析;C:谷子SiMAPK3结构域分析;D:谷子SiMAPK3三级结构模型;E:谷子SiMAPK3磷酸化位点预测
Fig. 1 Sequence and structure analysis of SiMAPK3 in foxtail millet A:Hydrophobicity of SiMAPK3. B:Analysis of the SiMAPK3 secondary structure. C:Analysis of the SiMAPK3 domain. D:The tertiary structure model of SiMAPK3. E:Prediction of phosphorylation sites in SiMAPK3

图2 SiMAPK3与其他物种MAPK蛋白的多重序列比对 红框表示高度保守的TEY特征序列
Fig. 2 Multiple sequence alignment of the SiMAPK3 and MAPK proteins from other species Red box indicates the specific sequence of highly conservative TEY
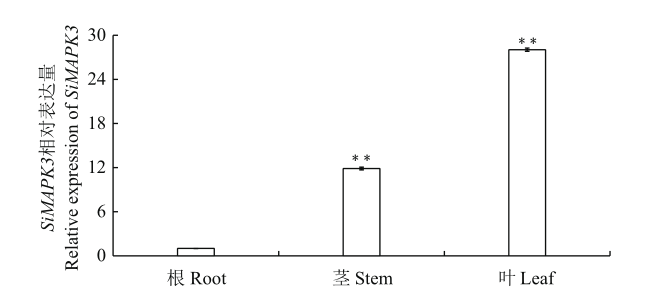
图4 谷子SiMAPK3基因在拔节前期各组织中的表达分析 RT-qPCR检测SiMAPK3基因的表达水平, 以谷子SiActin作为内参基因。n=3,**:极显著性差异(P<0.01)。下同
Fig. 4 Expression analysis of SiMAPK3 in different tissues of S. italica at the shooting stage of foxtail millet RT-qPCR analysis of SiMAPK3 gene expression in various organs, the SiActin in S. italica was used as an internal reference. n=3. Double asterisks(**)in each column indicate a significant difference at P<0.01 level. The same below
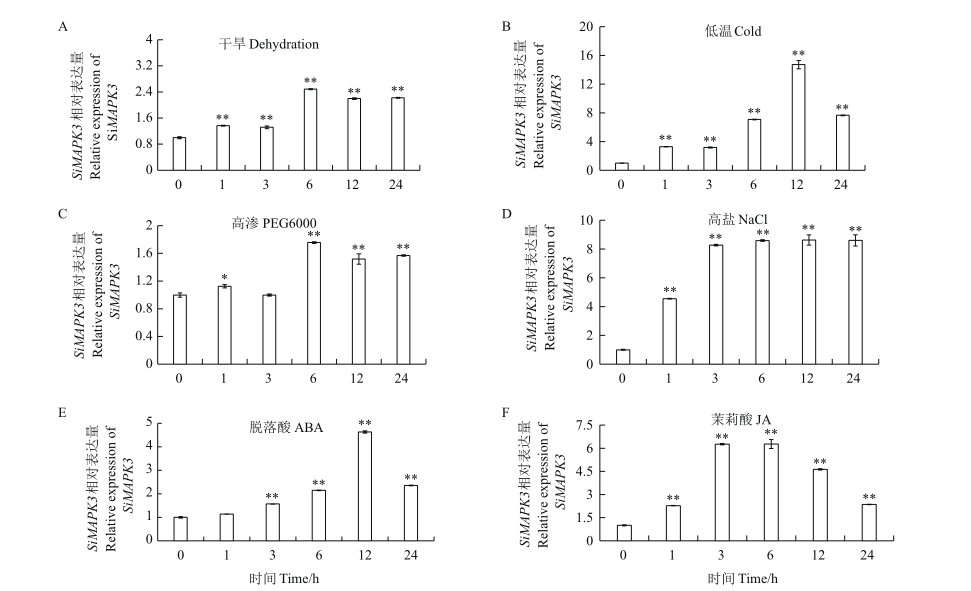
图5 SiMAPK3基因在不同处理下的表达模式 A:干旱胁迫下SiMAPK3基因的表达;B:低温胁迫下SiMAPK3基因的表达;C:20% PEG6000胁迫下SiMAPK3基因的表达;D:300 μmol/L高盐胁迫下SiMAPK3基因的表达;E:200 mmol/L脱落酸胁迫下SiMAPK3基因的表达;F:200 μmol/L 茉莉酸胁迫下SiMAPK3基因的表达。*:差异性显著(P<0.05)
Fig. 5 SiMAPK3 expression patterns under different treatments A: SiMAPK3 gene expression under dehydration; B: SiMAPK3 gene expression under 4℃; C: SiMAPK3 gene expression under 20% PEG6000; D: SiMAPK3 gene expression under 300 mmol/L NaCl; E: SiMAPK3 gene expression under 200 μmol/L ABA; F: SiMAPK3 gene expression under 200 μmol/L JA. Asterisk(*)indicates a difference at P<0.05 level
| [1] |
Xu J, Zhang SQ. Mitogen-activated protein kinase cascades in signaling plant growth and development[J]. Trends Plant Sci, 2015, 20(1): 56-64.
doi: 10.1016/j.tplants.2014.10.001 pmid: 25457109 |
| [2] |
Zhang MM, Zhang SQ. Mitogen-activated protein kinase cascades in plant signaling[J]. J Integr Plant Biol, 2022, 64(2): 301-341.
doi: 10.1111/jipb.13215 |
| [3] |
Chen XX, Ding YL, Yang YQ, et al. Protein kinases in plant responses to drought, salt, and cold stress[J]. J Integr Plant Biol, 2021, 63(1): 53-78.
doi: 10.1111/jipb.13061 |
| [4] |
Rodriguez MCS, Petersen M, Mundy J. Mitogen-activated protein kinase signaling in plants[J]. Annu Rev Plant Biol, 2010, 61: 621-649.
doi: 10.1146/annurev-arplant-042809-112252 pmid: 20441529 |
| [5] |
Zhang MM, Su JB, Zhang Y, et al. Conveying endogenous and exogenous signals: MAPK cascades in plant growth and defense[J]. Curr Opin Plant Biol, 2018, 45(Pt A): 1-10.
doi: S1369-5266(17)30213-3 pmid: 29753266 |
| [6] |
Ichimura K, Shinozaki K, Tena G, et al. Mitogen-activated protein kinase cascades in plants: a new nomenclature[J]. Trends Plant Sci, 2002, 7(7): 301-308.
doi: 10.1016/s1360-1385(02)02302-6 pmid: 12119167 |
| [7] |
Rao KP, Richa T, Kumar K, et al. In silico analysis reveals 75 members of mitogen-activated protein kinase kinase kinase gene family in rice[J]. DNA Res, 2010, 17(3): 139-153.
doi: 10.1093/dnares/dsq011 pmid: 20395279 |
| [8] |
Hamel LP, Nicole MC, Sritubtim S, et al. Ancient signals: comparative genomics of plant MAPK and MAPKK gene families[J]. Trends Plant Sci, 2006, 11(4): 192-198.
doi: 10.1016/j.tplants.2006.02.007 URL |
| [9] |
Jiang M, Chu ZQ. Comparative analysis of plant MKK gene family reveals novel expansion mechanism of the members and sheds new light on functional conservation[J]. BMC Genomics, 2018, 19(1): 407.
doi: 10.1186/s12864-018-4793-8 pmid: 29843611 |
| [10] |
Ichimura K, Mizoguchi T, Yoshida R, et al. Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6[J]. Plant J, 2000, 24(5): 655-665.
doi: 10.1046/j.1365-313x.2000.00913.x pmid: 11123804 |
| [11] |
Guo T, Lu ZQ, Shan JX, et al. ERECTA1 acts upstream of the OsMKKK10-OsMKK4-OsMPK6 cascade to control spikelet number by regulating cytokinin metabolism in rice[J]. Plant Cell, 2020, 32(9): 2763-2779.
doi: 10.1105/tpc.20.00351 URL |
| [12] |
Lee H, Jun YS, Cha OK, et al. Mitogen-activated protein kinases MPK3 and MPK6 are required for stem cell maintenance in the Arabidopsis shoot apical meristem[J]. Plant Cell Rep, 2019, 38(3): 311-319.
doi: 10.1007/s00299-018-2367-5 |
| [13] |
Zuch DT, Doyle SM, Majda M, et al. Cell biology of the leaf epidermis: fate specification, morphogenesis, and coordination[J]. Plant Cell, 2022, 34(1): 209-227.
doi: 10.1093/plcell/koab250 URL |
| [14] |
Melvin P, Prabhu SA, Veena M, et al. The pearl millet mitogen-activated protein kinase PgMPK4 is involved in responses to downy mildew infection and in jasmonic- and salicylic acid-mediated defense[J]. Plant Mol Biol, 2015, 87(3): 287-302.
doi: 10.1007/s11103-014-0276-8 pmid: 25527312 |
| [15] |
Zhang T, Schneider JD, Lin CW, et al. MPK4 phosphorylation dynamics and interacting proteins in plant immunity[J]. J Proteome Res, 2019, 18(3): 826-840.
doi: 10.1021/acs.jproteome.8b00345 pmid: 30632760 |
| [16] |
Jammes F, Song C, Shin D, et al. MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling[J]. Proc Natl Acad Sci USA, 2009, 106(48): 20520-20525.
doi: 10.1073/pnas.0907205106 pmid: 19910530 |
| [17] |
Yang XY, Wan ZW, Perry L, et al. Early millet use in Northern China[J]. Proc Natl Acad Sci USA, 2012, 109(10): 3726-3730.
doi: 10.1073/pnas.1115430109 pmid: 22355109 |
| [18] |
Diao XM, James S, Jeffrey LB, et al. Initiation of Setaria as a model plant[J]. Front Agr Sci Eng, 2014, 1(1): 16.
doi: 10.15302/J-FASE-2014011 URL |
| [19] |
Yang ZR, Zhang HS, Li XK, et al. A mini foxtail millet with an Arabidopsis-like life cycle as a C4 model system[J]. Nat Plants, 2020, 6(9): 1167-1178.
doi: 10.1038/s41477-020-0747-7 |
| [20] |
贾冠清, 刁现民. 中国谷子种业创新现状与未来展望[J]. 中国农业科学, 2022, 55(4): 653-665.
doi: 10.3864/j.issn.0578-1752.2022.04.003 |
|
Jia GQ, Diao XM. Current status and perspectives of innovation studies related to foxtail millet seed industry in China[J]. Sci Agric Sin, 2022, 55(4): 653-665.
doi: 10.3864/j.issn.0578-1752.2022.04.003 |
|
| [21] |
Zhao W, Zhang LL, Xu ZS, et al. Genome-wide analysis of MADS-box genes in foxtail millet(Setaria italica L.)and functional assessment of the role of SiMADS51 in the drought stress response[J]. Front Plant Sci, 2021, 12: 659474.
doi: 10.3389/fpls.2021.659474 URL |
| [22] |
Yang LY, Zhang Y, Guan RX, et al. Co-regulation of indole glucosinolates and camalexin biosynthesis by CPK5/CPK6 and MPK3/MPK6 signaling pathways[J]. J Integr Plant Biol, 2020, 62(11): 1780-1796.
doi: 10.1111/jipb.12973 |
| [23] |
Su JB, Yang LY, Zhu QK, et al. Active photosynthetic inhibition mediated by MPK3/MPK6 is critical to effector-triggered immunity[J]. PLoS Biol, 2018, 16(5): e2004122.
doi: 10.1371/journal.pbio.2004122 URL |
| [24] |
Shao YM, Yu XX, Xu XW, et al. The YDA-MKK4/MKK5-MPK3/MPK6 cascade functions downstream of the RGF1-RGI ligand-receptor pair in regulating mitotic activity in root apical meristem[J]. Mol Plant, 2020, 13(11): 1608-1623.
doi: 10.1016/j.molp.2020.09.004 pmid: 32916336 |
| [25] |
Rayapuram N, Bigeard J, Alhoraibi H, et al. Quantitative phosphoproteomic analysis reveals shared and specific targets of Arabidopsis mitogen-activated protein kinases(MAPKs)MPK3, MPK4, and MPK6[J]. Mol Cell Proteomics, 2018, 17(1): 61-80.
doi: 10.1074/mcp.RA117.000135 pmid: 29167316 |
| [26] | Yan ZW, Wang JX, Wang FX, et al. MPK3/6-induced degradation of ARR1/10/12 promotes salt tolerance in Arabidopsis[J]. EMBO Rep, 2021, 22(10): e52457. |
| [27] |
Liu YK, Liu LX, Qi JH, et al. Cadmium activates ZmMPK3-1 and ZmMPK6-1 via induction of reactive oxygen species in maize roots[J]. Biochem Biophys Res Commun, 2019, 516(3): 747-752.
doi: 10.1016/j.bbrc.2019.06.116 URL |
| [28] |
Wang NN, Li Y, Chen YH, et al. Phosphorylation of WRKY16 by MPK3-1 is essential for its transcriptional activity during fiber initiation and elongation in cotton(Gossypium hirsutum)[J]. Plant Cell, 2021, 33(8): 2736-2752.
doi: 10.1093/plcell/koab153 URL |
| [29] |
Long L, Xu FC, Zhao JR, et al. GbMPK3 overexpression increases cotton sensitivity to Verticillium dahliae by regulating salicylic acid signaling[J]. Plant Sci, 2020, 292: 110374.
doi: 10.1016/j.plantsci.2019.110374 URL |
| [30] |
Wu HJ, Si Q, Liu JM, et al. Regulation of Arabidopsis matrix metalloproteinases by mitogen-activated protein kinases and their function in leaf senescence[J]. Front Plant Sci, 2022, 13: 864986.
doi: 10.3389/fpls.2022.864986 URL |
| [31] |
Xin J, Li CL, Ning KX, et al. AtPFA-DSP3, an atypical dual-specificity protein tyrosine phosphatase, affects salt stress response by modulating MPK3 and MPK6 activity[J]. Plant Cell Environ, 2021, 44(5): 1534-1548.
doi: 10.1111/pce.v44.5 URL |
| [32] |
He CM, Liew LC, Yin LL, et al. The retrograde signaling regulator ANAC017 recruits the MKK9-MPK3/6, ethylene, and auxin signaling pathways to balance mitochondrial dysfunction with growth[J]. Plant Cell, 2022, 34(9): 3460-3481.
doi: 10.1093/plcell/koac177 URL |
| [1] | 王子颖, 龙晨洁, 范兆宇, 张蕾. 利用酵母双杂交系统筛选水稻中与OsCRK5互作蛋白[J]. 生物技术通报, 2023, 39(9): 117-125. |
| [2] | 刘雯锦, 马瑞, 刘升燕, 杨江伟, 张宁, 司怀军. 马铃薯StCIPK11的克隆及响应干旱胁迫分析[J]. 生物技术通报, 2023, 39(9): 147-155. |
| [3] | 陈晓, 于茗兰, 吴隆坤, 郑晓明, 逄洪波. 植物lncRNA及其对低温胁迫响应的研究进展[J]. 生物技术通报, 2023, 39(7): 1-12. |
| [4] | 丁凯鑫, 王立春, 田国奎, 王海艳, 李凤云, 潘阳, 庞泽, 单莹. 烯效唑缓解植物干旱损伤的研究进展[J]. 生物技术通报, 2023, 39(6): 1-11. |
| [5] | 韩华蕊, 杨宇琭, 门艺涵, 韩尚玲, 韩渊怀, 霍轶琼, 侯思宇. 基于代谢组学研究谷子SiYABBYs参与花发育过程中鼠李糖苷的生物合成[J]. 生物技术通报, 2023, 39(6): 189-198. |
| [6] | 王春语, 李政君, 王平, 张丽霞. 高粱表皮蜡质缺失突变体sb1抗旱生理生化分析[J]. 生物技术通报, 2023, 39(5): 160-167. |
| [7] | 刘辉, 卢扬, 叶夕苗, 周帅, 李俊, 唐健波, 陈恩发. 外源硫诱导苦荞镉胁迫响应的比较转录组学分析[J]. 生物技术通报, 2023, 39(5): 177-191. |
| [8] | 王琪, 胡哲, 富薇, 李光哲, 郝林. 伯克霍尔德氏菌GD17对黄瓜幼苗耐干旱的调节[J]. 生物技术通报, 2023, 39(3): 163-175. |
| [9] | 蒋路园, 丰美静, 杜雨晴, 邸葆, 陈段芬, 邱德有, 杨艳芳. 红豆杉低温半致死温度和低温胁迫下紫杉烷含量[J]. 生物技术通报, 2023, 39(3): 232-242. |
| [10] | 张晓燕, 杨淑华, 丁杨林. 植物感知和传递低温信号的分子机制[J]. 生物技术通报, 2023, 39(11): 28-35. |
| [11] | 于波, 秦晓惠, 赵杨. 植物感应干旱信号的机制[J]. 生物技术通报, 2023, 39(11): 6-17. |
| [12] | 邢媛, 宋健, 李俊怡, 郑婷婷, 刘思辰, 乔治军. 谷子AP基因家族鉴定及其对非生物胁迫的响应分析[J]. 生物技术通报, 2023, 39(11): 238-251. |
| [13] | 陈楚怡, 杨小梅, 陈胜艳, 陈斌, 岳莉然. ABA和干旱胁迫下菊花脑ZF-HD基因家族的表达分析[J]. 生物技术通报, 2023, 39(11): 270-282. |
| [14] | 冯策婷, 江律, 刘鑫颖, 罗乐, 潘会堂, 张启翔, 于超. 单叶蔷薇NAC基因家族鉴定及干旱胁迫响应分析[J]. 生物技术通报, 2023, 39(11): 283-296. |
| [15] | 毛可欣, 王海荣, 安淼, 刘腾飞, 王世金, 李健, 李国田. 中华猕猴桃GRAS基因家族鉴定及低温胁迫表达分析[J]. 生物技术通报, 2023, 39(11): 297-307. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||