








生物技术通报 ›› 2021, Vol. 37 ›› Issue (8): 46-54.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0734
袁恺1,2( ), 何伟1,2, 杨云丽3, 朱威宇1,2, 彭超1,2, 安泰1,2, 李丽3, 周卫强1,2(
), 何伟1,2, 杨云丽3, 朱威宇1,2, 彭超1,2, 安泰1,2, 李丽3, 周卫强1,2( )
)
收稿日期:2021-06-07
出版日期:2021-08-26
发布日期:2021-09-10
作者简介:袁恺,男,硕士,初级工程师,研究方向:生物化工;E-mail: 基金资助:
YUAN Kai1,2( ), HE Wei1,2, YANG Yun-li3, ZHU Wei-yu1,2, PENG Chao1,2, AN Tai1,2, LI Li3, ZHOU Wei-qiang1,2(
), HE Wei1,2, YANG Yun-li3, ZHU Wei-yu1,2, PENG Chao1,2, AN Tai1,2, LI Li3, ZHOU Wei-qiang1,2( )
)
Received:2021-06-07
Published:2021-08-26
Online:2021-09-10
摘要:
灵芝酸是药用灵芝菌的次级代谢产物,是灵芝菌的主要活性成分之一,已经在抗癌以及其他医药领域进行了深入研究。为了解决灵芝孢子、子实体生长周期长,生长环境要求高以及灵芝酸含量低等问题,灵芝菌深层液体发酵技术应运而生。尽管已经取得了一定的突破,但是发酵生产灵芝酸的低产量问题依然存在。灵芝酸生物合成途径的解析和调控对于解决发酵生产灵芝酸低产量问题意义重大,本文系统综述了近年来在灵芝酸生物合成和代谢调控方面的研究进展,并提出未来研究重点方向,为进一步通过基因工程手段和代谢通路调控手段实现灵芝酸的高浓度积累提供参考。
袁恺, 何伟, 杨云丽, 朱威宇, 彭超, 安泰, 李丽, 周卫强. 灵芝酸生物合成及代谢调控研究进展[J]. 生物技术通报, 2021, 37(8): 46-54.
YUAN Kai, HE Wei, YANG Yun-li, ZHU Wei-yu, PENG Chao, AN Tai, LI Li, ZHOU Wei-qiang. Research Progress on Biosynthesis and Metabolic Regulation of Ganoderic Acids[J]. Biotechnology Bulletin, 2021, 37(8): 46-54.

图1 灵芝酸生物合成途径 AACT:acetyl-CoA C-acyltransferase,乙酰辅酶A乙酰转移酶;HMGS:3-hydroxy-3-methylglutaryl-CoA synthase,3-羟基-3-甲基戊二酰CoA合酶;SE:squalene epoxidase,鲨烯环氧酶;CYP5150L8、CYP512U6:cytochromeP450,细胞色素P450单加氧酶;ERG27:3-keto sterol reductase,3-酮甾醇还原酶;HLDO:3-hydroxy-lanosta-8,24-dien-26-ol,3-羟基羊毛甾醇;HLDA:3-hydroxy-lanosta-8,24-dien-26-al,灵芝乙
Fig.1 Biosynthesis pathways of GAs
| 信号转导调控通路 Signal transduction regulatory pathway | 作用方式 Method of operation | 代谢水平变化 Changes in metabolic levels | 灵芝酸含量变化 Changes in ganoderic acid content | 文献 Reference |
|---|---|---|---|---|
| 钙调磷脂酶信号 | 添加10 mmol/L Ca2+ | cam、cna、crz1、hmgr、sqs、lss转录水平上调 | GAs含量增加3.7倍;灵芝酸MK、灵芝酸T、灵芝酸S和灵芝酸Me分别增加2.6倍、4.5倍、3.2倍和3.8倍 | [ |
| 添加10 mmol/L Mn2+ | cam、cna、crz1、pmr1、hmgr、sqs、lss转录水平上调 | GAs含量增加2.2倍 | [ | |
| 添加100 mmol/L Na+ | hmgr转录水平上调20倍,sqs上调109倍,lss上调4倍 | GAs含量增加2.8倍 | [ | |
| 活性氧信号 | 通过基因工程获得Glswi6沉默菌株 | NAHD氧化酶活性下降48%,hmgr、sqs、lss转录水平均下调 | GAs含量降低25% | [ |
| 在Glswi6沉默菌株发酵过程中回补1 mmol/L H2O2 | NAHD氧化酶活性和hmgr、sqs、lss转录水平恢复至野生型菌株水平 | GAs含量恢复至野生型菌株水平 | ||
| 通过基因工程获得Glskn7沉默菌株Skn7i-5和Skn7i-7 | H2O2含量上升73.7%和76.4%,ROS水平是原始菌株的1.78和1.82倍,hmgr转录水平上升56%、52%,sqs上调49%、53%,lss上调95%、93% | GAs含量分别提高52.1%和55.9% | [ | |
| 在Glskn7沉默菌株发酵过程中回补加入5 mmol/L N-乙酰半胱氨酸 | -- | GAs含量降低至与野生型菌株持平 | ||
| 通过基因工程获得PacC沉默菌株 | 胞内SOD、CAT、Gpx、Apx等抗氧化酶活性下降幅度超过40%,ROS水平上升幅度超过2倍 | GAs含量明显提升 | [ | |
| 当转录因子PacC沉默时,回补1 mmol/L NAC 和 2 mmol/L维生素C时 | -- | GAs含量降低至与出发菌株持平 | [ | |
| 细胞壁完整性信号 | 通过基因工程获得MAPK4沉默菌株 | hmgr、sqs、lss转录水平下调 | GAs含量最大降低40% | [ |
| 茉莉酮酸(JA)及其衍生物信号 | 添加254 μmol/L的MeJA | fps、sqs、hmgr、mvd、se转录水平上调 | GAs含量提高45.3% | [ |
| NO信号 | 添加5 mmol/L的NO供体 | sqs转录水平上调2.43倍 | 灵芝三帖含量提高40.94% | [ |
| cAMP信号 | 添加80 mmol/L咖啡因 | 尽管检测结果显示hmgr、sqs、lss转录水平下调,但是GAs的生物合成代谢表现出正向结果,目前作者仍在探究其中原因 | GAs含量增长3.6倍 | [ |
表1 灵芝酸生物合成途径的信号转导调控
Table 1 Signal transduction regulation of ganoderma acid biosynthesis pathway
| 信号转导调控通路 Signal transduction regulatory pathway | 作用方式 Method of operation | 代谢水平变化 Changes in metabolic levels | 灵芝酸含量变化 Changes in ganoderic acid content | 文献 Reference |
|---|---|---|---|---|
| 钙调磷脂酶信号 | 添加10 mmol/L Ca2+ | cam、cna、crz1、hmgr、sqs、lss转录水平上调 | GAs含量增加3.7倍;灵芝酸MK、灵芝酸T、灵芝酸S和灵芝酸Me分别增加2.6倍、4.5倍、3.2倍和3.8倍 | [ |
| 添加10 mmol/L Mn2+ | cam、cna、crz1、pmr1、hmgr、sqs、lss转录水平上调 | GAs含量增加2.2倍 | [ | |
| 添加100 mmol/L Na+ | hmgr转录水平上调20倍,sqs上调109倍,lss上调4倍 | GAs含量增加2.8倍 | [ | |
| 活性氧信号 | 通过基因工程获得Glswi6沉默菌株 | NAHD氧化酶活性下降48%,hmgr、sqs、lss转录水平均下调 | GAs含量降低25% | [ |
| 在Glswi6沉默菌株发酵过程中回补1 mmol/L H2O2 | NAHD氧化酶活性和hmgr、sqs、lss转录水平恢复至野生型菌株水平 | GAs含量恢复至野生型菌株水平 | ||
| 通过基因工程获得Glskn7沉默菌株Skn7i-5和Skn7i-7 | H2O2含量上升73.7%和76.4%,ROS水平是原始菌株的1.78和1.82倍,hmgr转录水平上升56%、52%,sqs上调49%、53%,lss上调95%、93% | GAs含量分别提高52.1%和55.9% | [ | |
| 在Glskn7沉默菌株发酵过程中回补加入5 mmol/L N-乙酰半胱氨酸 | -- | GAs含量降低至与野生型菌株持平 | ||
| 通过基因工程获得PacC沉默菌株 | 胞内SOD、CAT、Gpx、Apx等抗氧化酶活性下降幅度超过40%,ROS水平上升幅度超过2倍 | GAs含量明显提升 | [ | |
| 当转录因子PacC沉默时,回补1 mmol/L NAC 和 2 mmol/L维生素C时 | -- | GAs含量降低至与出发菌株持平 | [ | |
| 细胞壁完整性信号 | 通过基因工程获得MAPK4沉默菌株 | hmgr、sqs、lss转录水平下调 | GAs含量最大降低40% | [ |
| 茉莉酮酸(JA)及其衍生物信号 | 添加254 μmol/L的MeJA | fps、sqs、hmgr、mvd、se转录水平上调 | GAs含量提高45.3% | [ |
| NO信号 | 添加5 mmol/L的NO供体 | sqs转录水平上调2.43倍 | 灵芝三帖含量提高40.94% | [ |
| cAMP信号 | 添加80 mmol/L咖啡因 | 尽管检测结果显示hmgr、sqs、lss转录水平下调,但是GAs的生物合成代谢表现出正向结果,目前作者仍在探究其中原因 | GAs含量增长3.6倍 | [ |
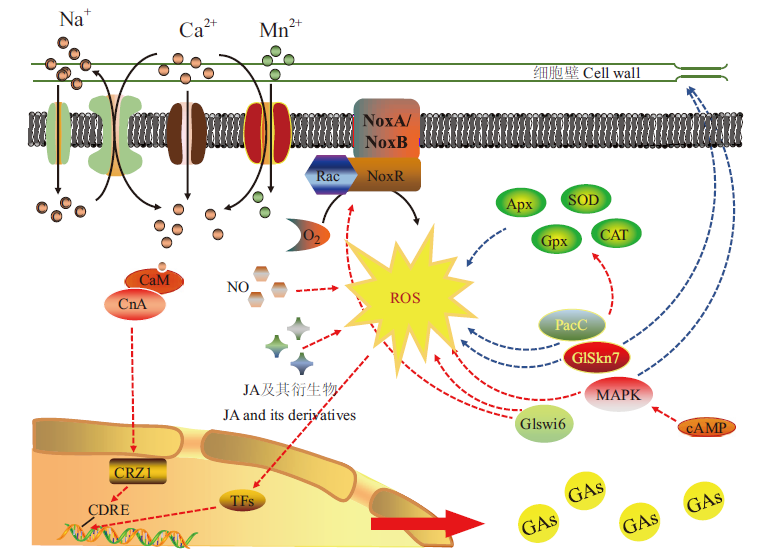
图2 灵芝菌胞内信号转导示意图 黑色实线表示移动路径,红色虚线表示具有促进作用,蓝色虚线表示具有抑制作用
Fig. 2 Schematic diagram of intracellular signal transduc-tion in G. lucidum The solid black line indicates the movement path,the red dashed line indicates that it has an accelerating effect,and the blue dashed line indicates that it has an inhibiting effect
| [1] | 赵艳. 灵芝关键成分三萜酸的深层发酵技术研究[D]. 长沙:中南林业科技大学, 2011. |
| Zhao Y. Submerged fermentation of key medicinal components, triterpene acids from Ganoderma lucidum[D]. Changsha:Central South University of Forestry & Technology, 2011. | |
| [2] | 刘维, 虎虓真, 朱莉, 等. 灵芝三萜的研究与应用进展[J]. 食品科学, 2019, 40(5):309-315. |
| Liu W, Hu XZ, Zhu L, et al. Recent progress in research and application of Ganoderma lucidum triterpenoids[J]. Food Sci, 2019, 40(5):309-315. | |
| [3] |
Kubota T, Asaka Y, Miura I, et al. Structures of ganoderic acid A and B, two new lanostane type bitter triterpenes from Ganoderma lucidum(FR. )KARST[J]. Helv Chim Acta, 1982, 65(2):611-619.
doi: 10.1002/(ISSN)1522-2675 URL |
| [4] |
Zhang W, Yu W, Ding X, et al. Self-assembled thermal gold nanorod-loaded thermosensitive liposome-encapsulated ganoderic acid for antibacterial and cancer photochemotherapy[J]. Artif Cells Nanomed Biotechnol, 2019, 47(1):406-419.
doi: 10.1080/21691401.2018.1559177 URL |
| [5] |
Liu Z, Li L, Xue B. Effect of ganoderic acid D on colon cancer Warburg effect:Role of SIRT3/cyclophilin D[J]. Eur J Pharmacol, 2018, 824:72-77.
doi: 10.1016/j.ejphar.2018.01.026 URL |
| [6] |
Sun ZW, Sun LB, Li WW. Effect of ganoderic acid on diethylnitrosamine-induced liver cancer in mice[J]. Trop J Pharm Res, 2021, 19(12):2639-2644.
doi: 10.4314/tjpr.v19i12.23 URL |
| [7] | Chi B, Wang S, Bi S, et al. Effects of ganoderic acid A on lipopolysaccharide-induced proinflammatory cytokine release from primary mouse microglia cultures[J]. Exp Ther Med, 2018, 15(1):847-853. |
| [8] |
Liang CY, Tian DN, Liu YZ, et al. Review of the molecular mechanisms of Ganoderma lucidum triterpenoids:Ganoderic acids A, C2, D, F, DM, X and Y[J]. Eur J Med Chem, 2019, 174:130-141.
doi: 10.1016/j.ejmech.2019.04.039 URL |
| [9] | 俞盈, 吴学谦, 熊科辉, 等. 灵芝三萜酸改善小鼠睡眠作用研究[J]. 食品工业科技, 2019, 40(10):297-301. |
| Yu Y, Wu XQ, Xiong KH, et al. Hypnotic effects of Ganoderma lucidum triterpene acids in mice[J]. Sci Technol Food Ind, 2019, 40(10):297-301. | |
| [10] |
Hossain A, Radwan FF, Doonan BP, et al. A possible cross-talk between autophagy and apoptosis in generating an immune response in melanoma[J]. Apoptosis, 2012, 17(10):1066-1078.
doi: 10.1007/s10495-012-0745-y pmid: 22847295 |
| [11] | 李丽, 杨云丽, 杨小凡, 等. 液体发酵生产灵芝三萜酸的过程调控研究进展[J]. 食品与发酵工业, 2021, 47(8):304-312. |
| Li L, Yang YL, Yang XF, et al. Advances on process regulation of Ganoderma triterpene acids production by liquid fermentation[J]. Food Ferment Ind, 2021, 47(8):304-312. | |
| [12] |
Li HJ, He YL, Zhang DH, et al. Enhancement of ganoderic acid production by constitutively expressing Vitreoscilla hemoglobin gene in Ganoderma lucidum[J]. J Biotechnol, 2016, 227:35-40.
doi: 10.1016/j.jbiotec.2016.04.017 URL |
| [13] |
Shiao MS. Natural products of the medicinal fungus Ganoderma lucidum:occurrence, biological activities, and pharmacological functions[J]. Chem Rec, 2003, 3(3):172-180.
doi: 10.1002/(ISSN)1528-0691 URL |
| [14] |
Chen S, Xu J, Liu C, et al. Genome sequence of the model medicinal mushroom Ganoderma lucidum[J]. Nat Commun, 2012, 3:913.
doi: 10.1038/ncomms1923 URL |
| [15] |
Cai SQ, Xiao H, Wang XZ, et al. Bioconversion of a ganoderic acid 3-hydroxy-lanosta-8, 24-Dien-26-oic acid by a crude enzyme from Ganoderma lucidum[J]. Process Biochem, 2020, 95:12-16.
doi: 10.1016/j.procbio.2020.05.002 URL |
| [16] |
Yang C, Li W, Li C, et al. Metabolism of ganoderic acids by a Ganoderma lucidum cytochrome P450 and the 3-keto sterol reductase ERG27 from yeast[J]. Phytochemistry, 2018, 155:83-92.
doi: 10.1016/j.phytochem.2018.07.009 URL |
| [17] |
Song X, Xiao H, Luo SW, et al. Biosynjournal of squalene-type triterpenoids in Saccharomyces cerevisiae by expression of CYP505D13 from Ganoderma lucidum[J]. Bioresour Bioprocess, 2019, 6(1):1-10.
doi: 10.1186/s40643-018-0235-3 URL |
| [18] |
Li HJ, He YL, Zhang DH, et al. Enhancement of ganoderic acid production by constitutively expressing Vitreoscilla hemoglobin gene in Ganoderma lucidum[J]. J Biotechnol, 2016, 227:35-40.
doi: 10.1016/j.jbiotec.2016.04.017 URL |
| [19] |
Xu JW, Xu YN, Zhong JJ. Enhancement of ganoderic acid accumulation by overexpression of an N-terminally truncated 3-hydroxy-3-methylglutaryl coenzyme A reductase gene in the basidiomycete Ganoderma lucidum[J]. Appl Environ Microbiol, 2012, 78(22):7968-7976.
doi: 10.1128/AEM.01263-12 URL |
| [20] | 张宗源. 组蛋白乙酰化调控灵芝酸与灵芝多糖生物合成代谢的研究[D]. 福州:福建师范大学, 2017. |
| Zhang ZY. Study on the regulation of histone acetylation on ganoderic acids and polysaccharide biosynthesis in Ganoderma lucidum[D]. Fuzhou:Fujian Normal University, 2017. | |
| [21] |
Fei Y, Li N, Zhang DH, et al. Increased production of ganoderic acids by overexpression of homologous farnesyl diphosphate synthase and kinetic modeling of ganoderic acid production in Ganoderma lucidum[J]. Microb Cell Factories, 2019, 18(1):115.
doi: 10.1186/s12934-019-1164-3 URL |
| [22] |
Ding YX, Ou-yang X, Shang CH, et al. Molecular cloning, characterization, and differential expression of a farnesyl-diphosphate synthase gene from the basidiomycetous fungus Ganoderma lucidum[J]. Biosci Biotechnol Biochem, 2008, 72(6):1571-1579.
doi: 10.1271/bbb.80067 URL |
| [23] |
Zhou JS, Ji SL, Ren MF, et al. Enhanced accumulation of individual ganoderic acids in a submerged culture of Ganoderma lucidum by the overexpression of squalene synthase gene[J]. Biochem Eng J, 2014, 90:178-183.
doi: 10.1016/j.bej.2014.06.008 URL |
| [24] |
Shang CH, Shi L, Ren A, et al. Molecular cloning, characterization, and differential expression of a lanosterol synthase gene from Ganoderma lucidum[J]. Biosci Biotechnol Biochem, 2010, 74(5):974-978.
doi: 10.1271/bbb.90833 URL |
| [25] |
Zhang DH, Li N, Yu X, et al. Overexpression of the homologous lanosterol synthase gene in ganoderic acid biosynjournal in Ganoderma lingzhi[J]. Phytochemistry, 2017, 134:46-53.
doi: S0031-9422(16)30256-4 pmid: 27894599 |
| [26] |
Paddon CJ, Westfall PJ, Pitera DJ, et al. High-level semi-synthetic production of the potent antimalarial artemisinin[J]. Nature, 2013, 496(7446):528-532.
doi: 10.1038/nature12051 URL |
| [27] |
Guo J, Zhou YJ, Hillwig ML, et al. CYP76AH1 catalyzes turnover of miltiradiene in tanshinones biosynjournal and enables heterologous production of ferruginol in yeasts[J]. PNAS, 2013, 110(29):12108-12113.
doi: 10.1073/pnas.1218061110 URL |
| [28] |
Dai Z, Wang B, Liu Y, et al. Producing aglycons of ginsenosides in bakers’ yeast[J]. Sci Rep, 2014, 4:3698.
doi: 10.1038/srep03698 URL |
| [29] |
Wang PP, Wei YJ, Fan Y, et al. Production of bioactive ginsenosides Rh2 and Rg3 by metabolically engineered yeasts[J]. Metab Eng, 2015, 29:97-105.
doi: 10.1016/j.ymben.2015.03.003 URL |
| [30] |
Wang WF, Xiao H, Zhong JJ. Biosynjournal of a ganoderic acid in Saccharomyces cerevisiae by expressing a cytochrome P450 gene from Ganoderma lucidum[J]. Biotechnol Bioeng, 2018, 115(7):1842-1854.
doi: 10.1002/bit.v115.7 URL |
| [31] |
Lan X, Yuan W, Wang M, et al. Efficient biosynjournal of antitumor ganoderic acid HLDOA using a dual tunable system for optimizing the expression of CYP5150L8 and a Ganoderma P450 reductase[J]. Biotechnol Bioeng, 2019, 116(12):3301-3311.
doi: 10.1002/bit.v116.12 URL |
| [32] |
Gu L, Zheng YM, Lian DH, et al. Production of triterpenoids from Ganoderma lucidum:Elicitation strategy and signal transduction[J]. Process Biochem, 2018, 69:22-32.
doi: 10.1016/j.procbio.2018.03.019 URL |
| [33] |
Gu L, Zhong X, Lian DH, et al. Triterpenoid biosynjournal and the transcriptional response elicited by nitric oxide in submerged fermenting Ganoderma lucidum[J]. Process Biochem, 2017, 60:19-26.
doi: 10.1016/j.procbio.2017.05.029 URL |
| [34] |
You BJ, Tien N, Lee MH, et al. Induction of apoptosis and ganoderic acid biosynjournal by cAMP signaling in Ganoderma lucidum[J]. Sci Rep, 2017, 7(1):318.
doi: 10.1038/s41598-017-00281-x URL |
| [35] |
Xu YN, Zhong JJ. Impacts of calcium signal transduction on the fermentation production of antitumor ganoderic acids by medicinal mushroom Ganoderma lucidum[J]. Biotechnol Adv, 2012, 30(6):1301-1308.
doi: 10.1016/j.biotechadv.2011.10.001 URL |
| [36] |
Xu YN, Xia XX, Zhong JJ. Induction of ganoderic acid biosynjournal by Mn2+ in static liquid cultivation of Ganoderma lucidum[J]. Biotechnol Bioeng, 2014, 111(11):2358-2365.
doi: 10.1002/bit.25288 URL |
| [37] |
Xu YN, Xia XX, Zhong JJ. Induced effect of Na(+)on ganoderic acid biosynjournal in static liquid culture of Ganoderma lucidum via calcineurin signal transduction[J]. Biotechnol Bioeng, 2013, 110(7):1913-1923.
doi: 10.1002/bit.24852 URL |
| [38] |
Zhang G, Ren A, Shi L, et al. Functional analysis of an APSES transcription factor(GlSwi6)involved in fungal growth, fruiting body development and ganoderic-acid biosynjournal in Ganoderma lucidum[J]. Microbiol Res, 2018, 207:280-288.
doi: 10.1016/j.micres.2017.12.015 URL |
| [39] |
Wang S, Shi L, Hu Y, et al. Roles of the Skn7 response regulator in stress resistance, cell wall integrity and GA biosynjournal in Ganoderma lucidum[J]. Fungal Genet Biol, 2018, 114:12-23.
doi: 10.1016/j.fgb.2018.03.002 URL |
| [40] | Wu FL, Zhang G, Ren A, et al. The pH-responsive transcription factor PacC regulates mycelial growth, fruiting body development, and ganoderic acid biosynjournal in Ganoderma lucidum[J]. Mycologia, 2016, 108(6):1104-1113. |
| [41] | Zhu J, Wu FL, Yue SN, et al. Functions of reactive oxygen species in apoptosis and ganoderic acid biosynjournal in Ganoderma lucidum[J]. FEMS Microbiol Lett, 2019, 366(23):fnaa015. |
| [42] | 李雄标. 灵芝促原活化蛋白激酶MAPK4和转录因子SWI6的功能研究[D]. 南京:南京农业大学, 2014. |
| Li XB. Functional analysis of mapkinase MAPK4 and transcription factor SWI6 in Ganoderma lucidum[D]. Nanjing:Nanjing Agricultural University, 2014. | |
| [43] |
Huang W, Shang YF, Chen PL, et al. MrpacC regulates sporulation, insect cuticle penetration and immune evasion in Metarhizium robertsii[J]. Environ Microbiol, 2015, 17(4):994-1008.
doi: 10.1111/1462-2920.12451 pmid: 24612440 |
| [44] |
Wu CG, Tian JL, Liu R, et al. Ornithine decarboxylase-mediated production of putrescine influences ganoderic acid biosynjournal by regulating reactive oxygen species in Ganoderma lucidum[J]. Appl Environ Microbiol, 2017, 83(20):e01289-17. DOI: 10.1128/aem.01289-17.
doi: 10.1128/aem.01289-17 |
| [45] |
Ren A, Shi L, Zhu J, et al. Shedding light on the mechanisms underlying the environmental regulation of secondary metabolite ganoderic acid in Ganoderma lucidum using physiological and genetic methods[J]. Fungal Genet Biol, 2019, 128:43-48.
doi: 10.1016/j.fgb.2019.03.009 URL |
| [46] |
Tripathi L, Wu LP, Chen JC, et al. Synjournal of Diblock copolymer poly-3-hydroxybutyrate-block-poly-3-hydroxyhexanoate[PHB-b-PHHx]by a β-oxidation weakened Pseudomonas putida KT2442[J]. Microb Cell Factories, 2012, 11(1):1-11.
doi: 10.1186/1475-2859-11-1 URL |
| [47] | 贺望兴. 灵芝全局性调控基因LaeA的克隆及其在灵芝酸生物合成过程中的差异表达[D]. 福州:福建师范大学, 2015. |
| He WX. Molecular cloning and differential gene expression of the global regulator LaeA in ganoderic acid biosynthesis process[D]. Fuzhou:Fujian Normal University, 2015. | |
| [48] | 孙超, 胡鸢雷, 徐江, 等. 灵芝:一种研究天然药物合成的模式真菌[J]. 中国科学:生命科学, 2013, 43(6):447-456. |
| Sun C, Hu YL, Xu J, et al. Ganoderma lucidum:an emerging medicinal model fungus for study of the biosynjournal of natural medicines[J]. Sci Sin:Vitae, 2013, 43(6):447-456. | |
| [49] | 张梦薇, 陈美榕, 刘舒雯, 等. LaeA在丝状真菌次级代谢、生长发育和其他重要生物过程中的作用[J]. 菌物学报, 2020, 39(3):548-555. |
| Zhang MW, Chen MR, Liu SW, et al. The roles of LaeA in secondary metabolism, growth and development and other important biological processes of filamentous fungi:a review[J]. Mycosystema, 2020, 39(3):548-555. | |
| [50] | 蓝丽雯. DNA甲基化调控灵芝酸生物合成代谢的研究[D]. 福州:福建师范大学, 2016. |
| Lan LW. Study on the regulation of DNA methylation on ganoderic acids biosynthesis in Ganoderma lucidum[D]. Fuzhou:Fujian Normal University, 2016. | |
| [51] | 张宗源, 蒋咏梅, 章文贤. 组蛋白乙酰化对灵芝生长、灵芝多糖和灵芝酸生物合成的影响[J]. 中国农业科学, 2020, 53(3):632-641. |
| Zhang ZY, Jiang YM, Zhang WX. Effects of histone acetylation on Ganoderma lucidum growth, polysaccharide and ganoderic acid biosynjournal[J]. Sci Agric Sin, 2020, 53(3):632-641. |
| [1] | 叶云芳, 田清尹, 施婷婷, 王亮, 岳远征, 杨秀莲, 王良桂. 植物中β-紫罗兰酮生物合成及调控研究进展[J]. 生物技术通报, 2023, 39(8): 91-105. |
| [2] | 成婷, 苑帅, 张晓元, 林良才, 李欣, 张翠英. 酿酒酵母异丁醇合成途径调控的研究进展[J]. 生物技术通报, 2023, 39(7): 80-90. |
| [3] | 马芳芳, 刘冠闻, 庞冰, 蒋春美, 师俊玲. 强化细胞外排提高工程菌类黄酮产量的策略[J]. 生物技术通报, 2023, 39(5): 63-76. |
| [4] | 王祥锟, 宋学宏, 刘金龙, 郭培红, 庄晓峰, 韦良孟, 周凡, 张树宇, 高攀攀, 魏凯. 新型冠状病毒亚单位疫苗研制及其高效免疫增强剂的筛选[J]. 生物技术通报, 2023, 39(1): 305-314. |
| [5] | 段玥彤, 王鹏年, 张春宝, 林春晶. 植物黄烷酮-3-羟化酶基因研究进展[J]. 生物技术通报, 2022, 38(6): 27-33. |
| [6] | 徐重新, 张霄, 刘媛, 仲建锋, 谢雅晶, 卢莉娜, 高美静, 刘贤金. 靶向模拟Bt Cry1C蛋白抗虫功能的人源化基因工程抗体筛选及鉴定[J]. 生物技术通报, 2022, 38(5): 191-200. |
| [7] | 李毅丹, 单晓辉. 赤霉素代谢调控与绿色革命[J]. 生物技术通报, 2022, 38(2): 195-204. |
| [8] | 马艳琴, 邱益彬, 李莎, 徐虹. 透明质酸的生物合成及其代谢工程的研究进展[J]. 生物技术通报, 2022, 38(2): 252-262. |
| [9] | 田清尹, 岳远征, 申慧敏, 潘多, 杨秀莲, 王良桂. 植物观赏器官中类胡萝卜素代谢调控的研究进展[J]. 生物技术通报, 2022, 38(12): 35-46. |
| [10] | 马勤, 雷瑞峰, 迪力热巴·阿不都肉苏力, 穆耶赛尔·奥斯曼, 祖力胡玛尔·肉孜, 安登第. 环境胁迫下内生菌与宿主代谢相互作用研究进展[J]. 生物技术通报, 2021, 37(3): 153-161. |
| [11] | 孟晓建, 于建东, 郑小梅, 郑平, 李志敏, 孙际宾, 叶勤. 小分子代谢物对黑曲霉己糖激酶和丙酮酸激酶的酶活调控[J]. 生物技术通报, 2021, 37(12): 180-190. |
| [12] | 胡濒月, 胡杨, 成文敏, 赵素梅, 赵红业, 魏红江. Leptin过表达对猪前脂肪细胞脂滴形成的研究[J]. 生物技术通报, 2020, 36(8): 111-119. |
| [13] | 陈慧玲, 张青云, 孙凯. 漆酶介导生物体内酚类氧化偶联的基本原理及其在绿色合成中的应用[J]. 生物技术通报, 2020, 36(5): 193-204. |
| [14] | 郭振强, 张勇, 曹运齐, 刘云云, 赵于, 吴蔼民. 燃料乙醇发酵技术研究进展[J]. 生物技术通报, 2020, 36(1): 238-244. |
| [15] | 何虎翼, 唐洲萍, 杨鑫, 樊吴静, 谭冠宁, 李丽淑, 何新民. 马铃薯淀粉合成与降解研究进展[J]. 生物技术通报, 2019, 35(4): 101-107. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||