








Biotechnology Bulletin ›› 2026, Vol. 42 ›› Issue (1): 329-337.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0794
Previous Articles Next Articles
LI Zheng1( ), QIU Wei-yue1, SUN Rui-xue1, ZHAO Xiao1,2,3(
), QIU Wei-yue1, SUN Rui-xue1, ZHAO Xiao1,2,3( )
)
Received:2025-07-24
Online:2026-01-26
Published:2026-02-04
Contact:
ZHAO Xiao
E-mail:2826290073@qq.com;zhaoxiao1@hbut.edu.cn
LI Zheng, QIU Wei-yue, SUN Rui-xue, ZHAO Xiao. Overexpression of the xylR Gene Driven by Different Promoters Enhances Glucose-xylose Co-utilization Capability in Escherichia coli[J]. Biotechnology Bulletin, 2026, 42(1): 329-337.
引物名称 Primer name | 序列 Sequence (5´-3´) |
|---|---|
| P1 | AGGCCCTTTCGTCTTCAAGACGCATATGCTGGATCCTT |
| P2 | CATACACGGTGCCTGACTGCGCTACAACATGACCTCGCTATTTA |
| P3 | TCTTGAAGACGAAAGGGCCTC |
| P4 | GCAGTCAGGCACCGTGTA |
| P5 | ATGTTTACTAAACGTCACCGCA |
| P6 | CAGGATAACCAACGGTTAATCG |
| P7 | CTGATTCTGTGGATAACCGT |
| P8 | CGGTGACGTTTAGTAAACATACTAGTATTATACCTAGGACTGAGC |
| P9 | AGGCCCTTTCGTCTTCAAGACTGCAGTCCTGAAGCTTG |
| P10 | CATACACGGTGCCTGACTGCCATCGTCAGTATTGACTGCAG |
| P11 | AGTGTGAAAGCTGACAACC |
| P12 | GCACTCGAAGATACGGAT |
| P13 | TGCCGGTTAATTACTAAGGG |
| P14 | GACAGCTAGCTCAGTCCTA |
| P15 | TATAGCGCTAGCAGCACG |
| QxylA-F | GAAACCGCCTGCTTTGAGAA |
| QxylA-R | CATTGAAGCTAACCACGCGA |
| QxylB-F | GATTATTGTGTGGCGTACGC |
| QxylB-R | TACATAGCTTTTGCCATGCG |
| QxylR-F | TGGGGATTATTGTGTGGCGT |
| QxylR-R | CATAGCTTTTGCCATGCGCT |
| QxylF-F | GGTGTCGATGTTCTTGTCAT |
| QxylF-R | CTTGGCGTTGTTATCTACCG |
| QxylG-F | TATGACCTGATGACGCTACG |
| QxylG-R | TGCTCAGTTAATGAGGCTGT |
| QxylH-F | TTGCAGCTATCATCGCAATC |
| QxylH-R | CACAATGATGGTAAGTGGCA |
| PcysG-F | TGGGCCAGGTAGCGAAATAC |
| PcysG-R | TAGGCAGAGCAACCAGAAGC |
Table 1 Primers used and their sequences
引物名称 Primer name | 序列 Sequence (5´-3´) |
|---|---|
| P1 | AGGCCCTTTCGTCTTCAAGACGCATATGCTGGATCCTT |
| P2 | CATACACGGTGCCTGACTGCGCTACAACATGACCTCGCTATTTA |
| P3 | TCTTGAAGACGAAAGGGCCTC |
| P4 | GCAGTCAGGCACCGTGTA |
| P5 | ATGTTTACTAAACGTCACCGCA |
| P6 | CAGGATAACCAACGGTTAATCG |
| P7 | CTGATTCTGTGGATAACCGT |
| P8 | CGGTGACGTTTAGTAAACATACTAGTATTATACCTAGGACTGAGC |
| P9 | AGGCCCTTTCGTCTTCAAGACTGCAGTCCTGAAGCTTG |
| P10 | CATACACGGTGCCTGACTGCCATCGTCAGTATTGACTGCAG |
| P11 | AGTGTGAAAGCTGACAACC |
| P12 | GCACTCGAAGATACGGAT |
| P13 | TGCCGGTTAATTACTAAGGG |
| P14 | GACAGCTAGCTCAGTCCTA |
| P15 | TATAGCGCTAGCAGCACG |
| QxylA-F | GAAACCGCCTGCTTTGAGAA |
| QxylA-R | CATTGAAGCTAACCACGCGA |
| QxylB-F | GATTATTGTGTGGCGTACGC |
| QxylB-R | TACATAGCTTTTGCCATGCG |
| QxylR-F | TGGGGATTATTGTGTGGCGT |
| QxylR-R | CATAGCTTTTGCCATGCGCT |
| QxylF-F | GGTGTCGATGTTCTTGTCAT |
| QxylF-R | CTTGGCGTTGTTATCTACCG |
| QxylG-F | TATGACCTGATGACGCTACG |
| QxylG-R | TGCTCAGTTAATGAGGCTGT |
| QxylH-F | TTGCAGCTATCATCGCAATC |
| QxylH-R | CACAATGATGGTAAGTGGCA |
| PcysG-F | TGGGCCAGGTAGCGAAATAC |
| PcysG-R | TAGGCAGAGCAACCAGAAGC |
Fig. 1 Verification electrophoresis profile of the expressing plasmidsA: Electrophoretogram of plasmid CX-322 (1: JH15; 2: CX-322). B: Electrophoretogram of plasmid RX-322 (1: JH15; 2: RX-322). C: Electrophoretogram of plasmid JX-322 (1: JH15; 2: JX-322). D: Electrophoretogram of plasmid PX-322 (1: JH15; 2: PX-322). M: DL5000 DNA marker
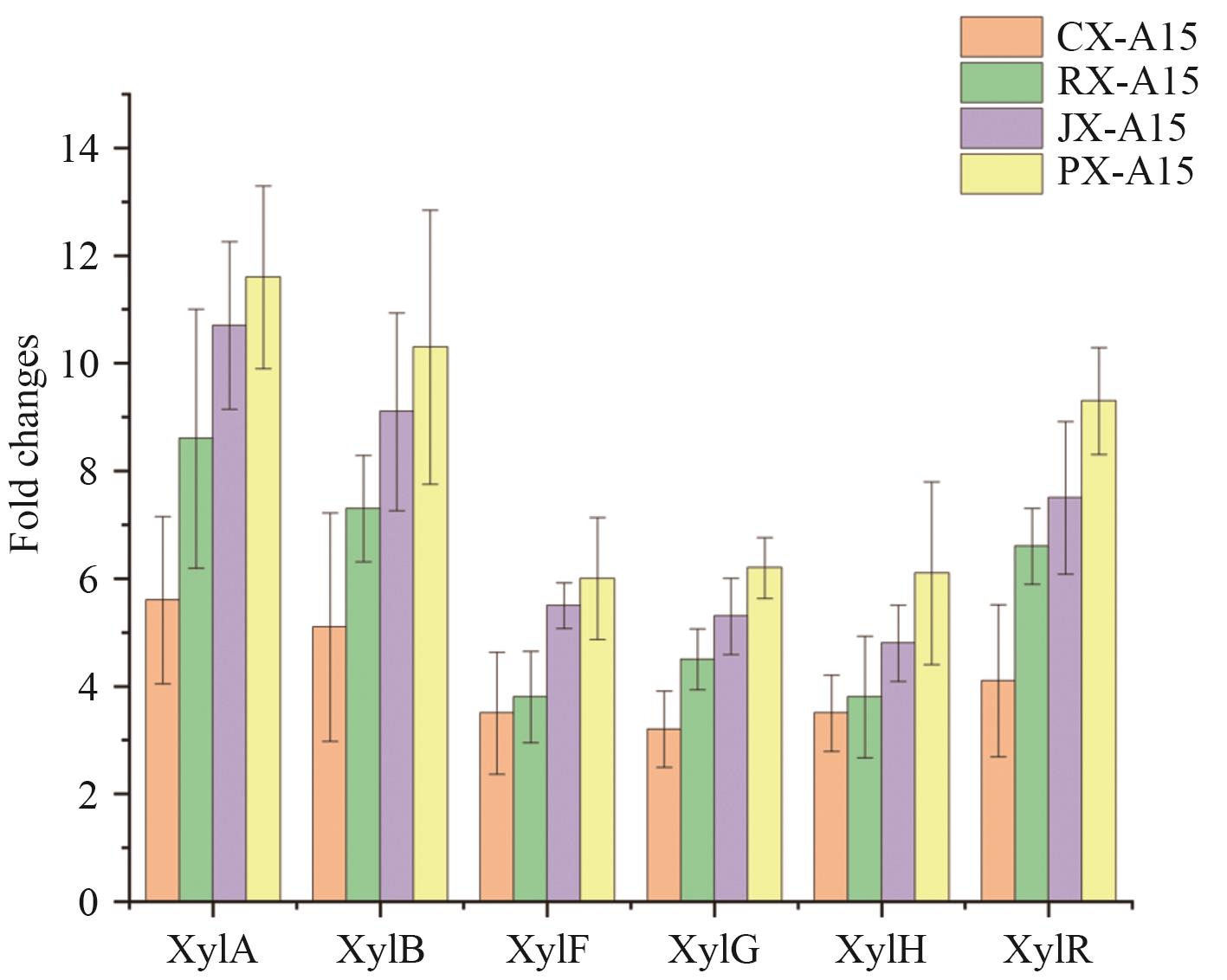
Fig. 3 Differential analysis of the transcription levels of xylose transport and metabolism genesA: Fold change (FC)>2 is considered as significant difference
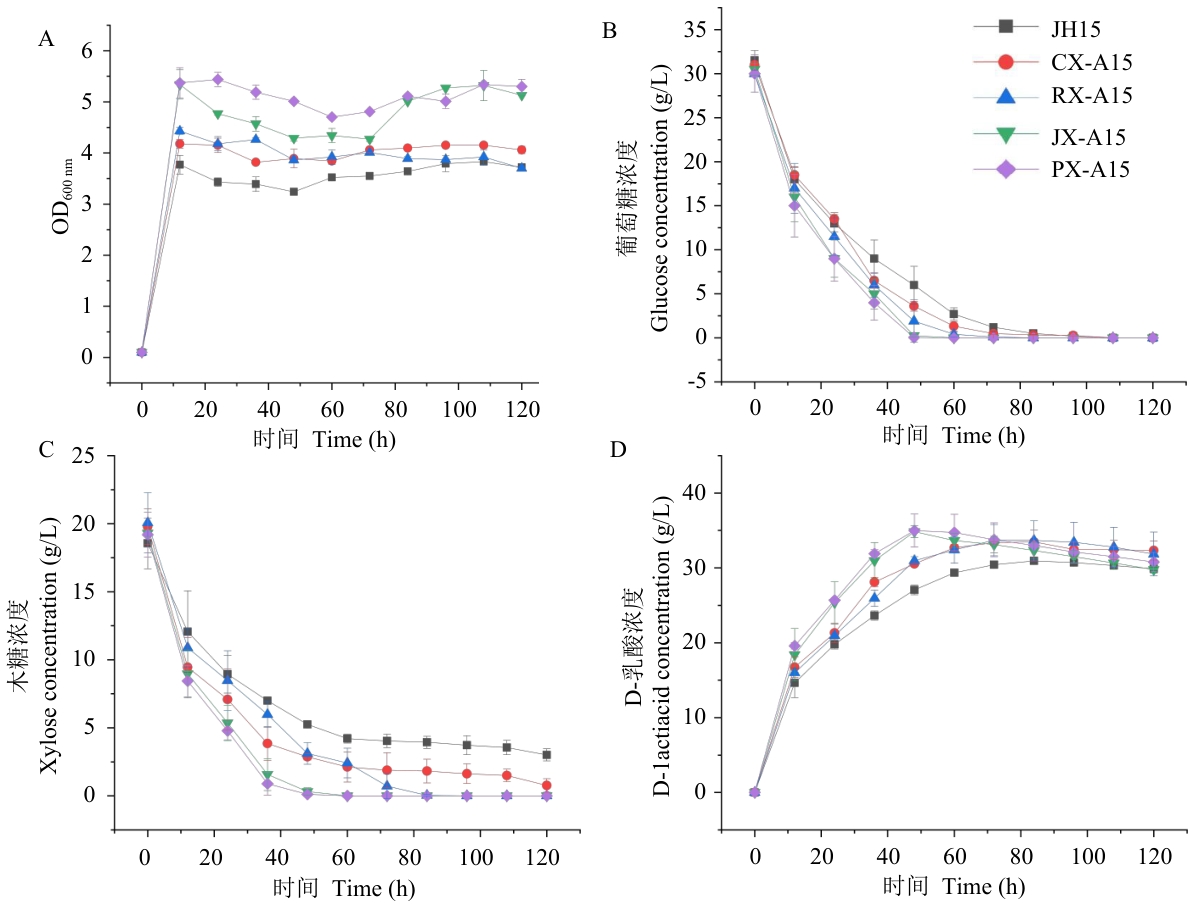
Fig. 4 Fermentation result curves of starting strain JH15 and recombinant strains CX-A15, RX-A15, JX-A15, and PX-A15 in mixed sugars (3% glucose+2% xylose)A: Growth curves of different strains. B: Glucose consumption curves of different strains. C: Xylose consumption curves of different strains. D: D-lactic acid production curves of different strains
发酵菌株 Fermentative strain | 葡萄糖消耗速率 Glucose consumption rate (g/(L·h)) | 木糖消耗速率Xylose consumption rate (g/(L·h)) | D-乳酸生产速率 D-lactic acid production rate (g/(L·h)) | 糖酸转化率 Sugar-acid conversion rate (%) | 最大OD600 nm Maximum OD600 nm | 发酵周期 Fermentation cycle (h) |
|---|---|---|---|---|---|---|
| JH15 | 0.369±0.01c | 0.129±0.01c | 0.368±0c | 64b | 3.77c | 84 |
| CX-A15 | 0.424±0c | 0.158±0c | 0.399±0c | 64b | 4.18bc | 84 |
| RX-A15 | 0.493±0.02b | 0.238±0.02b | 0.468±0.02b | 63b | 4.43b | 72 |
| JX-A15 | 0.630±0.01a | 0.396±0.01a | 0.726±0.01a | 66a | 5.34a | 48 |
| PX-A15 | 0.624±0.03a | 0.398±0.02a | 0.729±0.03a | 66a | 5.44a | 48 |
Table 2 Fermentation results of the original strain JH15 and recombinant strain CX-A15, RX-A15, JX-A15, PX-A15 in fermentation with mixed-sugars (3% glucose + 2% xylose)
发酵菌株 Fermentative strain | 葡萄糖消耗速率 Glucose consumption rate (g/(L·h)) | 木糖消耗速率Xylose consumption rate (g/(L·h)) | D-乳酸生产速率 D-lactic acid production rate (g/(L·h)) | 糖酸转化率 Sugar-acid conversion rate (%) | 最大OD600 nm Maximum OD600 nm | 发酵周期 Fermentation cycle (h) |
|---|---|---|---|---|---|---|
| JH15 | 0.369±0.01c | 0.129±0.01c | 0.368±0c | 64b | 3.77c | 84 |
| CX-A15 | 0.424±0c | 0.158±0c | 0.399±0c | 64b | 4.18bc | 84 |
| RX-A15 | 0.493±0.02b | 0.238±0.02b | 0.468±0.02b | 63b | 4.43b | 72 |
| JX-A15 | 0.630±0.01a | 0.396±0.01a | 0.726±0.01a | 66a | 5.34a | 48 |
| PX-A15 | 0.624±0.03a | 0.398±0.02a | 0.729±0.03a | 66a | 5.44a | 48 |
| [1] | 赵烁, 李莹, 王震, 等. 木质纤维素预处理方法的研究进展 [J]. 中国造纸, 2024, 43(8): 29-38. |
| Zhao S, Li Y, Wang Z, et al. Research progress of lignocellulose pretreatment methods [J]. China Pulp Pap, 2024, 43(8): 29-38. | |
| [2] | Zuroff TR, Curtis WR. Developing symbiotic consortia for lignocellulosic biofuel production [J]. Appl Microbiol Biotechnol, 2012, 93(4): 1423-1435. |
| [3] | Gao BY, Sun CR, Yang T, et al. Biphasic solvent systems enabled lignocellulosic biomass fractionation: a pathway towards comprehensive biomass utilization [J]. Ind Crops Prod, 2023, 202: 117036. |
| [4] | Kim JH, Block DE, Mills DA. Simultaneous consumption of pentose and hexose sugars: an optimal microbial phenotype for efficient fermentation of lignocellulosic biomass [J]. Appl Microbiol Biotechnol, 2010, 88(5): 1077-1085. |
| [5] | Luo YE, Zhang T, Wu H. The transport and mediation mechanisms of the common sugars in Escherichia coli [J]. Biotechnol Adv, 2014, 32(5): 905-919. |
| [6] | 刘倩钰, 吴丽雯, 牛建军, 等. 细菌磷酸转移酶系统(PTS)的组成与功能研究进展 [J]. 微生物学通报, 2020, 47(7): 2266-2277. |
| Liu QY, Wu LW, Niu JJ, et al. Research progress of the composition and function of bacterial phosphotransferase system [J]. Microbiol China, 2020, 47(7): 2266-2277. | |
| [7] | Wang YX, Cao LF, Bi MY, et al. Wobble editing of cre-box by unspecific CRISPR/Cas9 causes CCR release and phenotypic changes in Bacillus pumilus [J]. Front Chem, 2021, 9: 717609. |
| [8] | Kaplan NA, Islam KN, Kanis FC, et al. Simultaneous glucose and xylose utilization by an Escherichia coli catabolite repression mutant [J]. Appl Environ Microbiol, 2024, 90(2): e02169-23. |
| [9] | Song S, Park C. Organization and regulation of the D-xylose operons in Escherichia coli K-12: XylR acts as a transcriptional activator [J]. J Bacteriol, 1997, 179(22): 7025-7032. |
| [10] | Ni LS, Tonthat NK, Chinnam N, et al. Structures of the Escherichia coli transcription activator and regulator of diauxie, XylR: an AraC DNA-binding family member with a LacI/GalR ligand-binding domain [J]. Nucleic Acids Res, 2013, 41(3): 1998-2008. |
| [11] | Fu HX, Zhang HH, Guo XL, et al. Elimination of carbon catabolite repression in Clostridium tyrobutyricum for enhanced butyric acid production from lignocellulosic hydrolysates [J]. Bioresour Technol, 2022, 357: 127320. |
| [12] | Heo JM, Kim HJ, Lee SJ. Efficient anaerobic consumption of D-xylose by E. coli BL21(DE3) via xylR adaptive mutation [J]. BMC Microbiol, 2021, 21(1): 332. |
| [13] | Yuan DX, Liu BB, Jiang L, et al. XylR overexpression in Escherichia coli alleviated transcriptional repression by Arabinose and enhanced xylitol bioproduction from xylose mother liquor [J]. Appl Biochem Biotechnol, 2024, 196(10): 6624-6637. |
| [14] | 沐万孟, 朱莺莺, 孟佳炜, 等. 一种高产L-岩藻糖的重组大肠杆菌的构建方法及应用: CN115960812A [P]. 2023-04-14. |
| Mu WM, Zhu YY, Meng JW, et al. A method for constructing and application of recombinant Escherichia coli for high-yield production of L-fucose: CN115960812A [P]. 2023-04-14. | |
| [15] | Doi Y, Ikegami Y. Pyruvate formate-lyase is essential for fumarate-independent anaerobic glycerol utilization in the Enterococcus faecalis strain W11 [J]. J Bacteriol, 2014, 196(13): 2472-2480. |
| [16] | Lu HY, Zhao X, Wang YZ, et al. Enhancement of D-lactic acid production from a mixed glucose and xylose substrate by the Escherichia coli strain JH15 devoid of the glucose effect [J]. BMC Biotechnol, 2016, 16(1): 19. |
| [17] | 彭雷, 赵艳, 马银花. 基于巢式PCR的重叠延伸PCR优化 [J]. 安徽农业科学, 2016, 44(20): 126-127. |
| Peng L, Zhao Y, Ma YH. Optimization of splicing by overlap extension PCR using nested PCR [J]. J Anhui Agric Sci, 2016, 44(20): 126-127. | |
| [18] | Kragl U, Kruse W, Hummel W, et al. Enzyme engineering aspects of biocatalysis: Cofactor regeneration as example [J]. Biotechnol Bioeng, 1996, 52(2): 309-319. |
| [19] | Zhou K, Zhou LH, Lim Q, et al. Novel reference genes for quantifying transcriptional responses of Escherichia coli to protein overexpression by quantitative PCR [J]. BMC Mol Biol, 2011, 12(1): 18. |
| [20] | 朱捷, 葛奉娟, 王欲晓. 改进的双波长法测定二元混合体系中葡萄糖和木糖含量 [J]. 安徽农业科学, 2016, 44(3): 9-12. |
| Zhu J, Ge FJ, Wang YX. Detection of glucose and xylose in duplex mixture by improved dual-wavelength spectrophotometry [J]. J Anhui Agric Sci, 2016, 44(3): 9-12. | |
| [21] | 谢莹莹, 张悦, 刘雁鸣, 等. HPLC法同时测定乳酸中L-乳酸及6种有机酸杂质的含量 [J]. 中南药学, 2022, 20(10): 2390-2393. |
| Xie YY, Zhang Y, Liu YM, et al. Simultaneous determination of L-lactic acid and 6 organic acid impurities in lactic acid with HPLC [J]. Cent South Pharm, 2022, 20(10): 2390-2393. | |
| [22] | Sievert C, Nieves LM, Panyon LA, et al. Experimental evolution reveals an effective avenue to release catabolite repression via mutations in XylR [J]. Proc Natl Acad Sci U S A, 2017, 114(28): 7349-7354. |
| [23] | Barthe M, Tchouanti J, Gomes PH, et al. Availability of the molecular switch XylR controls phenotypic heterogeneity and lag duration during Escherichia coli adaptation from glucose to xylose [J]. mBio, 2020, 11(6) |
| [24] | Oh SJ, Lee HJ, Hwang JH, et al. Validating a xylose regulator to increase polyhydroxybutyrate production for utilizing mixed sugars from lignocellulosic biomass using Escherichia coli [J]. J Microbiol Biotechnol, 2024, 34(3): 700-709. |
| [25] | Molina-Vázquez ER, Caspeta L, Gosset G, et al. Tailoring Escherichia coli BL21 (DE3) for preferential xylose utilization via metabolic and regulatory engineering [J]. Appl Microbiol Biotechnol, 2025, 109(1): 54. |
| [26] | 丁小云, 顾健健, 王永泽, 等. 产D-乳酸重组大肠杆菌ptsG基因的敲除及其混合糖同步发酵 [J]. 生物技术通报, 2015, 31(12): 221-226. |
| Ding XY, Gu JJ, Wang YZ, et al. The knockout of gene ptsG of recombinant Escherichia coli producing D-lactic acid and the simultaneous fermentation of mixed sugars [J]. Biotechnol Bull, 2015, 31(12): 221-226. | |
| [27] | Maeda M, Shimada T, Ishihama A. Strength and regulation of seven rRNA promoters in Escherichia coli [J]. PLoS One, 2015, 10(12): e0144697. |
| [28] | Peng S, Stephan R, Hummerjohann J, et al. Evaluation of three reference genes of Escherichia coli for mRNA expression level normalization in view of salt and organic acid stress exposure in food [J]. FEMS Microbiol Lett, 2014, 355(1): 78-82. |
| [29] | Teh MY, Ooi KH, Danny Teo SX, et al. An expanded synthetic biology toolkit for gene expression control in Acetobacteraceae [J]. ACS Synth Biol, 2019, 8(4): 708-723. |
| [30] | Zhou SD, Iverson AG, Grayburn WS. Doubling the catabolic reducing power (NADH) output of Escherichia coli fermentation for production of reduced products [J]. Biotechnol Prog, 2010, 26(1): 45-51. |
| [31] | Fox KJ, Prather KL. Carbon catabolite repression relaxation in Escherichia coli: global and sugar-specific methods for glucose and secondary sugar co-utilization [J]. Curr Opin Chem Eng, 2020, 30: 9-16. |
| [1] | ZHANG Chi-hao, LIU Jin-nan, CHAO Yue-hui. Cloning and Functional Analysis of a bZIP Transcription Factor MtbZIP29 from Medicago truncatula [J]. Biotechnology Bulletin, 2026, 42(1): 241-250. |
| [2] | YAN Meng-yang, LIANG Xiao-yang, DAI Jun-ang, ZHANG Yan, GUAN Tuan, ZHANG Hui, LIU Liang-bo, SUN Zhi-hua. Screening of Amoxicillin-degrading Bacteria and Study on Its Degradation Mechanisms [J]. Biotechnology Bulletin, 2025, 41(9): 314-325. |
| [3] | FU Bo-han, MAO Hua, ZHAO Xin-cheng, LU Hong, OU Yong-bin, YAO Yin-an. Cloning of SOS1 Gene Promoters from Poplar and Analysis of Its Response to Salt Stress [J]. Biotechnology Bulletin, 2025, 41(7): 205-213. |
| [4] | ZHANG Ze, YANG Xiu-li, NING Dong-xian. Identification of 4CL Gene Family in Arachis hypogaea L. and Expression Analysis in Response to Drought and Salt Stress [J]. Biotechnology Bulletin, 2025, 41(7): 117-127. |
| [5] | HUANG Xu-sheng, ZHOU Ya-li, CHAI Xu-dong, WEN Jing, WANG Ji-ping, JIA Xiao-yun, LI Run-zhi. Cloning of Plastidial PfLPAT1B Gene from Perilla frutescens and Its Functional Analysis in Oil Biosynthesis [J]. Biotechnology Bulletin, 2025, 41(7): 226-236. |
| [6] | LIANG Li-cun, WANG Ke-fen, SONG Zu-huan, LIU Meng-ting, LI Jia-yu, LUO Hui-ying, YAO Bin, YANG Hao-meng. Improving the Efficiency of Gene Editing by Optimizing sgRNA in Aspergillus tubingensis [J]. Biotechnology Bulletin, 2025, 41(3): 62-70. |
| [7] | LIN Zi-yi, WU Yi-zhou, YE Fang-xian, ZHU Shu-ying, LIU Yan-min, LIU Su-shuang. Functional Analysis of Soybean GmPM31 Gene Promoter Involvement in Response to High Temperature and Humidity Stress [J]. Biotechnology Bulletin, 2025, 41(3): 90-97. |
| [8] | XUE Xiao-bin, NING Lin, ZHOU Yu, LIU Hong-jun, GAO Zhao-zu, WANG Zhen-ping, LI Dong-mei. Identification of VvOMTs Gene Families and Functional Analysis of Promoters in Wine Grapes [J]. Biotechnology Bulletin, 2025, 41(12): 168-176. |
| [9] | MAO Li-jing, JIN Xiao-xuan, SHI Wan-ting, HU Fei-yang, ZHANG Yuan-rong, XIONG Liang-bin, REN Lu. Modifying the Probiotic Escherichia coli Nissle 1917 for the Biosynthesis of Indirubin via Metabolic Engineering [J]. Biotechnology Bulletin, 2025, 41(12): 74-81. |
| [10] | ZHOU Si-yan, DING Wei-quan, DONG Ping, WENG Han-zhi, XU Rui-rui, KANG Zhen. Advances in Synthetic Biology Platform Development and Application for Escherichiacoli Nissle 1917 [J]. Biotechnology Bulletin, 2025, 41(11): 47-61. |
| [11] | WEI Min-hua, LI Xiao-tong, JIANG Ya-wen, ZHOU Piao-piao, WANG Kai, SUN Hao, LU Nan, ZHANG Cheng-lin. Systems Metabolic Engineering for Highly Efficient L-isoleucine Production in Escherichia coli [J]. Biotechnology Bulletin, 2025, 41(11): 110-120. |
| [12] | YANG Yi-chen, ZHU Hong-yu, SU Xiao-yun, WANG Yuan, LUO Hui-ying, TIAN Jian, YAO Bin, HUANG Huo-qing, ZHANG Jie. Construction of an Efficient Microbial Cell Factory for Inositol Production from Glucose-fructose Syrup [J]. Biotechnology Bulletin, 2025, 41(11): 121-133. |
| [13] | HE Ting-yu, PANG Yu, ZHANG Yuan-yang, SUN Xue, LI Yu, LU Fu-ping, LI Qing-gang. Construction of a High-production Lacto -N-triose Ⅱ-producing Escherichia coli Strain [J]. Biotechnology Bulletin, 2025, 41(11): 143-152. |
| [14] | RAO Jun, ZHAO Chen, LI Duan-hua, LIAO Hao, HUANG Jia-yu, WANG Lu. Application of Auto-induction Strategy in Ergothioneine Biosynthesis [J]. Biotechnology Bulletin, 2025, 41(1): 333-346. |
| [15] | ZHANG Jing-an, HU Xiao-long, CAO Bei-bei, LIAO Min, SHU Chang-long, ZHANG Jie, WANG Kui, CAO Hai-qun. Construction and Characterization of Rapid Visual Expression Vector for Bacillus thuringiensis [J]. Biotechnology Bulletin, 2025, 41(1): 95-102. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||