








Biotechnology Bulletin ›› 2025, Vol. 41 ›› Issue (1): 347-356.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0675
Previous Articles Next Articles
PEI Xu-juan( ), DI Jing-yi, LIU Hao, GAO Wei-xia(
), DI Jing-yi, LIU Hao, GAO Wei-xia( )
)
Received:2024-07-15
Online:2025-01-26
Published:2025-01-22
Contact:
GAO Wei-xia
E-mail:13652151929@163.com;gaoweixia@tust.edu.cn
PEI Xu-juan, DI Jing-yi, LIU Hao, GAO Wei-xia. Exploration of Regulatory Elements for Hyaluronic Acid Molecular Weight in Streptococcus zooepidemicus via Transcriptome Analysis[J]. Biotechnology Bulletin, 2025, 41(1): 347-356.
| Type | Laboratory number | Genotype/Phenotype | Description | Source |
|---|---|---|---|---|
| Plasmid | pLH67 | pSET4s::sacB | Temperature sensitive vector | Our Laboratory |
| pLH421 | pDL278 | Streptococcus shuttle vector, Specr | Our Laboratory | |
| pXJ00 | pLH421::PR22 | pDL278 carrying PR22 | Our Laboratory | |
| pXJ01 | pSET4s::sacB::arcA LR | arcA markerless deletion vector | This work | |
| pXJ02 | pLH421::PR22::arcA | pDL278 carrying PR22::arcA gene | This work | |
| pXJ03 | pSET4s::sacB::argF LR | argF markerless deletion vector | This work | |
| pXJ04 | pLH421::PR22::argF | pDL278 carrying PR22::argF gene | This work | |
| Strains | S12 | ATCC 39920 | Wild-type strain | Our Laboratory |
| S3896 | ΔarcA | arcA markerless deletion mutant | This work | |
| S3897 | arcA/OP | S12 containing plasmid pXJ02 | This work | |
| S3898 | ΔargF | argF markerless deletion mutant | This work | |
| S3899 | argF/OP | S12 containing plasmid pXJ04 | This work |
Table 1 Plasmids and strains used in this study
| Type | Laboratory number | Genotype/Phenotype | Description | Source |
|---|---|---|---|---|
| Plasmid | pLH67 | pSET4s::sacB | Temperature sensitive vector | Our Laboratory |
| pLH421 | pDL278 | Streptococcus shuttle vector, Specr | Our Laboratory | |
| pXJ00 | pLH421::PR22 | pDL278 carrying PR22 | Our Laboratory | |
| pXJ01 | pSET4s::sacB::arcA LR | arcA markerless deletion vector | This work | |
| pXJ02 | pLH421::PR22::arcA | pDL278 carrying PR22::arcA gene | This work | |
| pXJ03 | pSET4s::sacB::argF LR | argF markerless deletion vector | This work | |
| pXJ04 | pLH421::PR22::argF | pDL278 carrying PR22::argF gene | This work | |
| Strains | S12 | ATCC 39920 | Wild-type strain | Our Laboratory |
| S3896 | ΔarcA | arcA markerless deletion mutant | This work | |
| S3897 | arcA/OP | S12 containing plasmid pXJ02 | This work | |
| S3898 | ΔargF | argF markerless deletion mutant | This work | |
| S3899 | argF/OP | S12 containing plasmid pXJ04 | This work |
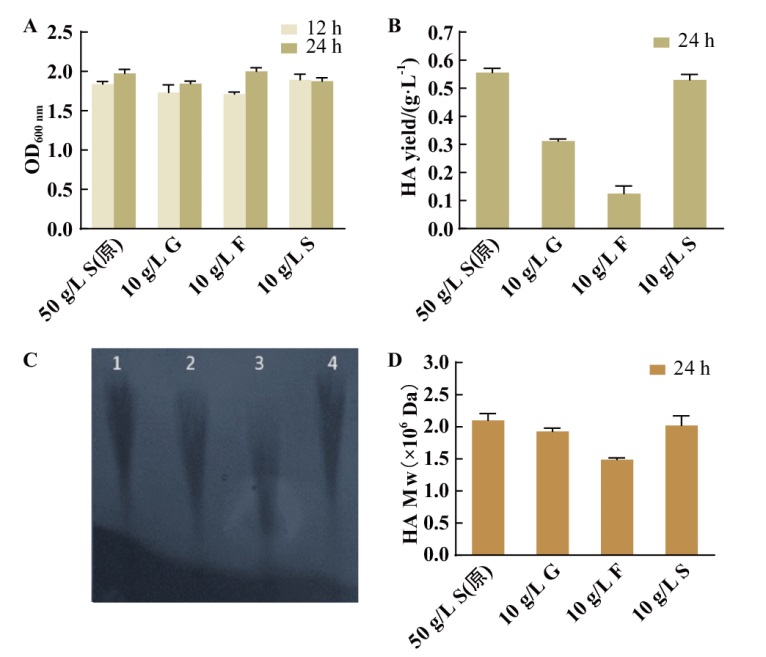
Fig. 2 Characterization of HA production by wild-type strains under different carbon sources A: Growth of wild-type strain in shaking bottle. B: Yield of hyaluronic acid from wild-type strain in shaking flask for 24 h. C: Molecular weight gel map of 24 h HA, 1: original medium(50 g/L sucrose); 2: 10 g/L glucose; 3: 10 g/L fructose; 4: 10 g/L sucrose). D: HPGPC to detect HA molecular weight of fermented S12 strain under different carbon sources. In the figure, 50 g/L S(original medium): 50 g/L sucrose(original medium); 10 g/L G: 10 g/L glucose; 10 g/L F: 10 g/L fructose; 10 g/L S: 10 g/L sucrose
| Gene | Gene ID | 对数期(FPKM) | Regulate | |
|---|---|---|---|---|
| S12(50 g /L蔗糖)S12(50 g/L sucrose) | S12(10 g /L果糖)S12(10 g/L fructose) | |||
| arcA | SeseC_RS02770 | 102 | 1 700 | Up |
| argF | SeseC _RS02780 | 160 | 1 900 | Up |
| scrA | SeseC _RS01805 | 1 907.5 | 20 | Down |
| fruR2 | SeseC _RS05815 | 1.72 | 175 | Up |
| SeseC _RS05805 | SeseC _RS05805 | 20.25 | 364.3 | Up |
| SeseC _RS01810 | SeseC _RS01810 | 344 | 8.2 | Down |
Table 2 Key genes with distinct transcriptome differences
| Gene | Gene ID | 对数期(FPKM) | Regulate | |
|---|---|---|---|---|
| S12(50 g /L蔗糖)S12(50 g/L sucrose) | S12(10 g /L果糖)S12(10 g/L fructose) | |||
| arcA | SeseC_RS02770 | 102 | 1 700 | Up |
| argF | SeseC _RS02780 | 160 | 1 900 | Up |
| scrA | SeseC _RS01805 | 1 907.5 | 20 | Down |
| fruR2 | SeseC _RS05815 | 1.72 | 175 | Up |
| SeseC _RS05805 | SeseC _RS05805 | 20.25 | 364.3 | Up |
| SeseC _RS01810 | SeseC _RS01810 | 344 | 8.2 | Down |
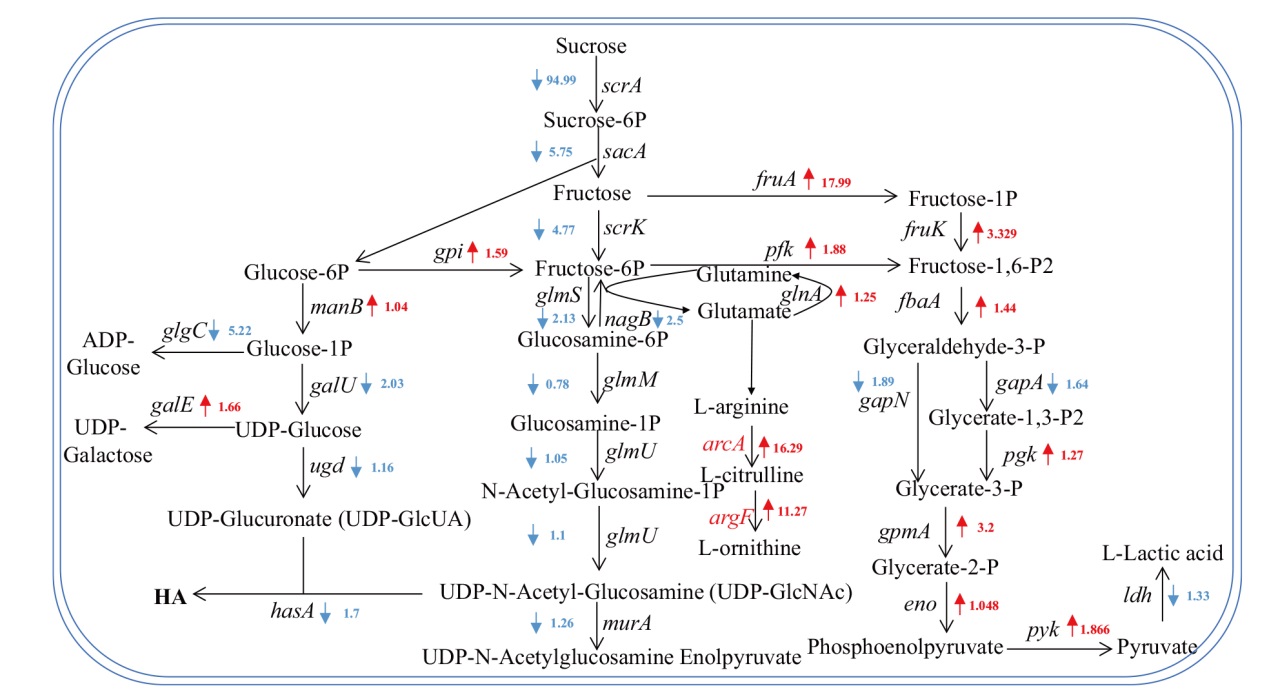
Fig. 6 Schematic diagram of transcriptional levels of genes related to sucrose metabolism pathway in Streptococcus zooepi-demicus Red indicates elevated gene transcription levels, and blue indicates decreased gene transcription levels
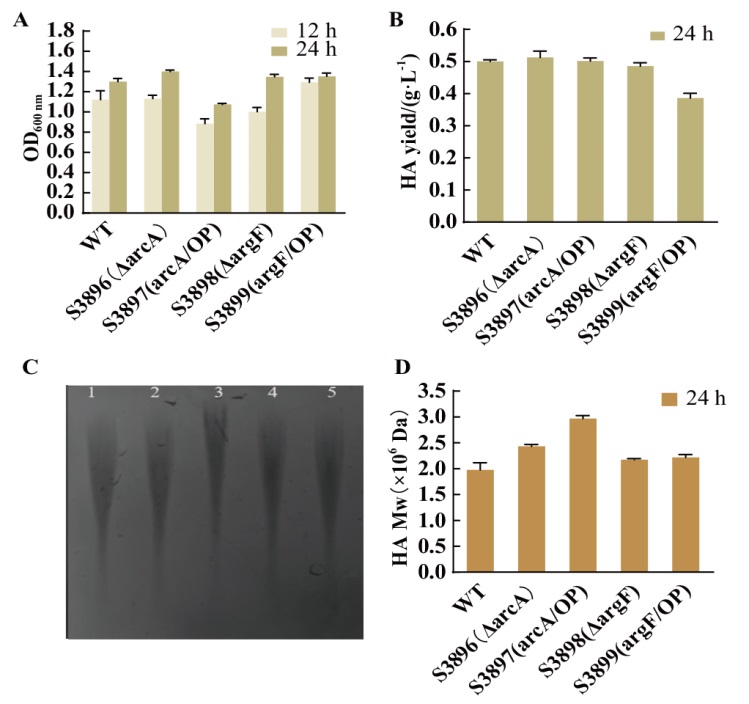
Fig. 7 Characterization of HA in CDM medium of four constructed strains and wild-type strains A: Growth of each strain in shaking flask. B: HA production of each strain in shaking flask for 24 h. C: Molecular weight gel map of HA at 24 h (1: Streptococcus zooepidemicus wild-type S12; 2: ΔarcA strain; 3: arcA/OP strain; 4: ΔargF strain; 5: argF/OP strain). D: HPGPC to detect the molecular weight of HA produced by each strain
| [1] | Zheng XL, Wang BT, Tang X, et al. Absorption, metabolism, and functions of hyaluronic acid and its therapeutic prospects in combination with microorganisms: a review[J]. Carbohydr Polym, 2023, 299: 120153. |
| [2] | Yao ZY, Qin JF, Gong JS, et al. Versatile strategies for bioproduction of hyaluronic acid driven by synthetic biology[J]. Carbohydr Polym, 2021, 264: 118015. |
| [3] | Snetkov P, Zakharova K, Morozkina S, et al. Hyaluronic acid: the influence of molecular weight on structural, physical, physico-chemical, and degradable properties of biopolymer[J]. Polymers, 2020, 12(8): 1800. |
| [4] |
Puré E, Assoian RK. Rheostatic signaling by CD44 and hyaluronan[J]. Cell Signal, 2009, 21(5): 651-655.
doi: 10.1016/j.cellsig.2009.01.024 pmid: 19174187 |
| [5] |
Kang Z, Zhou ZX, Wang Y, et al. Bio-based strategies for producing glycosaminoglycans and their oligosaccharides[J]. Trends Biotechnol, 2018, 36(8): 806-818.
doi: S0167-7799(18)30094-5 pmid: 29674113 |
| [6] | Cheng FY, Gong QY, Yu HM, et al. High-titer biosynthesis of hyaluronic acid by recombinant Corynebacterium glutamicum[J]. Biotechnol J, 2016, 11(4): 574-584. |
| [7] |
Ke CL, Sun LP, Qiao DL, et al. Antioxidant acitivity of low molecular weight hyaluronic acid[J]. Food Chem Toxicol, 2011, 49(10): 2670-2675.
doi: 10.1016/j.fct.2011.07.020 pmid: 21787831 |
| [8] | Kim M, Yang H, Kim H, et al. Novel cosmetic patches for wrinkle improvement: retinyl retinoate- and ascorbic acid-loaded dissolving microneedles[J]. Int J Cosmet Sci, 2014, 36(3): 207-212. |
| [9] |
Waddell DD, Bert JM. The use of hyaluronan after arthroscopic surgery of the knee[J]. Arthroscopy, 2010, 26(1): 105-111.
doi: 10.1016/j.arthro.2009.05.009 pmid: 20117634 |
| [10] | Henrotin Y, Bannuru R, Malaise M, et al. Hyaluronan derivative HYMOVIS® increases cartilage volume and type II collagen turnover in osteoarhritic knee: data from MOKHA study[J]. BMC Musculoskelet Disord, 2019, 20(1): 293. |
| [11] |
Litwiniuk M, Krejner A, Speyrer MS, et al. Hyaluronic acid in inflammation and tissue regeneration[J]. Wounds, 2016, 28(3): 78-88.
pmid: 26978861 |
| [12] |
Frenkel JS. The role of hyaluronan in wound healing[J]. Int Wound J, 2014, 11(2): 159-163.
doi: 10.1111/j.1742-481X.2012.01057.x pmid: 22891615 |
| [13] | Yamasaki K, Drolle E, Nakagawa H, et al. Impact of a low molecular weight hyaluronic acid derivative on contact lens wettability[J]. Cont Lens Anterior Eye, 2021, 44(3): 101334. |
| [14] | Huang WC, Chen SJ, Chen TL. Production of hyaluronic acid by repeated batch fermentation[J]. Biochem Eng J, 2008, 40(3): 460-464. |
| [15] | Hartman AH, Liu HL, Melville SB. Construction and characterization of a lactose-inducible promoter system for controlled gene expression in Clostridium perfringens[J]. Appl Environ Microbiol, 2011, 77(2): 471-478. |
| [16] | Chen SJ, Chen JL, Huang WC, et al. Fermentation process development for hyaluronic acid production by Streptococcus zooepidemicus ATCC 39920[J]. Korean J Chem Eng, 2009, 26(2): 428-432. |
| [17] | Liu L, Yang HQ, Zhang DX, et al. Enhancement of hyaluronic acid production by batch culture of Streptococcus zooepidemicus with n-dodecane as an oxygen vector[J]. J Microbiol Biotechnol, 2009, 19(6): 596-603. |
| [18] | Johns MR, Goh LT, Oeggerli A. Effect of pH, agitation and aeration on hyaluronic acid production by Streptococcus zooepidemicus[J]. Biotechnol Lett, 1994, 16(5): 507-512. |
| [19] | Kim JH, Yoo SJ, Oh DK, et al. Selection of a Streptococcus equi mutant and optimization of culture conditions for the production of high molecular weight hyaluronic acid[J]. Enzyme Microb Technol, 1996, 19(6): 440-445. |
| [20] | Chen WY, Marcellin E, Steen JA, et al. The role of hyaluronic acid precursor concentrations in molecular weight control in Streptococ-cus zooepidemicus[J]. Mol Biotechnol, 2014, 56(2): 147-156. |
| [21] | Chen WY, Marcellin E, Hung J, et al. Hyaluronan molecular weight is controlled by UDP-N-acetylglucosamine concentration in Strep-tococcus zooepidemicus[J]. J Biol Chem, 2009, 284(27): 18007-18014. |
| [22] | Jeeva P, Jayaprakash SR, Jayaraman G. Hyaluronic acid production is enhanced by harnessing the heme-induced respiration in recombinant Lactococcus lactis cultures[J]. Biochem Eng J, 2022, 182: 108428. |
| [23] |
Sheng JZ, Ling PX, Zhu XQ, et al. Use of induction promoters to regulate hyaluronan synthase and UDP-glucose-6-dehydrogenase of Streptococcus zooepidemicus expression in Lactococcus lac-tis: a case study of the regulation mechanism of hyaluronic acid polymer[J]. J Appl Microbiol, 2009, 107(1): 136-144.
doi: 10.1111/j.1365-2672.2009.04185.x pmid: 19302304 |
| [24] |
Jokela TA, Jauhiainen M, Auriola S, et al. Mannose inhibits hyaluronan synthesis by down-regulation of the cellular pool of UDP-N-acetylhexosamines[J]. J Biol Chem, 2008, 283(12): 7666-7673.
doi: 10.1074/jbc.M706001200 pmid: 18201970 |
| [25] | Jagannath S, Ramachandran KB. Influence of competing metabolic processes on the molecular weight of hyaluronic acid synthesized by Streptococcus zooepidemicus[J]. Biochem Eng J, 2010, 48(2): 148-158. |
| [26] |
Weigel PH, DeAngelis PL. Hyaluronan synthases: a decade-plus of novel glycosyltransferases[J]. J Biol Chem, 2007, 282(51): 36777-36781.
doi: 10.1074/jbc.R700036200 pmid: 17981795 |
| [27] | Wang J, He W, Wang T, et al. Sucrose-modified iron nanoparticles for highly efficient microbial production of hyaluronic acid by Streptococcus zooepidemicus[J]. Colloids Surf B Biointerfaces, 2021, 205: 111854-111862. |
| [28] | Marques WL, Raghavendran V, Stambuk BU, et al. Sucrose and Saccharomyces cerevisiae: a relationship most sweet[J]. FEMS Yeast Res, 2016, 16(1): fov107. |
| [29] |
van de Rijn I, Kessler RE. Growth characteristics of group A streptococci in a new chemically defined medium[J]. Infect Immun, 1980, 27(2): 444-448.
doi: 10.1128/iai.27.2.444-448.1980 pmid: 6991416 |
| [30] | Armstrong DC, Cooney MJ, Johns MR. Growth and amino acid requirements of hyaluronic-acid-producing Streptococcus zooepidemi-cus[J]. Appl Microbiol Biotechnol, 1997, 47(3): 309-312. |
| [31] | 孙晓燕. 兽疫链球菌基因敲除系统的建立及hasE功能研究[D]. 天津: 天津科技大学, 2014. |
| Sun XY. Establishment of gene knockout system of Streptococcus zooepidemicus and study on HasE function[D]. Tianjin: Tianjin University of Science & Technology, 2014. | |
| [32] | Oueslati N, Leblanc P, Harscoat-Schiavo C, et al. CTAB turbidimetric method for assaying hyaluronic acid in complex environments and under cross-linked form[J]. Carbohydr Polym, 2014, 112: 102-108. |
| [33] |
Funderburgh JL, Chandler JW. An agarose gel electrophoretic method for analysis of sulfated glycosaminoglycans of cultured cells[J]. Anal Biochem, 1978, 91(2): 464-472.
pmid: 9762132 |
| [34] |
Tlapak-Simmons VL, Baggenstoss BA, Kumari K, et al. Kinetic characterization of the recombinant hyaluronan synthases from Streptococcus pyogenes and Streptococcus equisimilis[J]. J Biol Chem, 1999, 274(7): 4246-4253.
doi: 10.1074/jbc.274.7.4246 pmid: 9933624 |
| [35] |
Marcellin E, Chen WY, Nielsen LK. Understanding plasmid effect on hyaluronic acid molecular weight produced by Streptococcus equi subsp. zooepidemicus[J]. Metab Eng, 2010, 12(1): 62-69.
doi: 10.1016/j.ymben.2009.09.001 pmid: 19782148 |
| [36] | Badle SS, Jayaraman G, Ramachandran KB. Ratio of intracellular precursors concentration and their flux influences hyaluronic acid molecular weight in Streptococcus zooepidemicus and recombinant Lactococcus lactis[J]. Bioresour Technol, 2014, 163: 222-227. |
| [37] | Xu MJ, Rao ZM, Dou WF, et al. Site-directed mutagenesis and feedback-resistant N-acetyl-L-glutamate kinase(NAGK)increase Corynebacterium crenatum L-arginine production[J]. Amino Acids, 2012, 43(1): 255-266. |
| [38] | Ikeda M, Mitsuhashi S, Tanaka K, et al. Reengineering of a Corynebacterium glutamicum L-arginine and L-citrulline producer[J]. Appl Environ Microbiol, 2009, 75(6): 1635-1641. |
| [39] |
Wray LV Jr, Ferson AE, Rohrer K, et al. TnrA, a transcription factor required for global nitrogen regulation in Bacillus subtilis[J]. Proc Natl Acad Sci USA, 1996, 93(17): 8841-8845.
pmid: 8799114 |
| [1] | KOU Bei-sen, CHENG Meng-meng, GUO Xue-qin, GE Bin, LIU Di, LU Hai, LI Hui. Effects of Histone Deacetylase Inhibitor TSA Treatment on the Stem Development of Poplar [J]. Biotechnology Bulletin, 2025, 41(1): 240-251. |
| [2] | NIE Zhu-xin, GUO Jin, QIAO Zi-yang, LI Wei-wei, ZHANG Xue-yan, LIU Chun-yang, WANG Jing. Transcriptome Analysis of the Anthocyanin Biosynthesis in the Fruit Development Processes of Lycium ruthenicum Murr. [J]. Biotechnology Bulletin, 2024, 40(8): 106-117. |
| [3] | ZHOU Lin, HUANG Shun-man, SU Wen-kun, YAO Xiang, QU Yan. Identification of the bHLH Gene Family and Selection of Genes Related to Color Formation in Camellia reticulata [J]. Biotechnology Bulletin, 2024, 40(8): 142-151. |
| [4] | GAO Meng-meng, ZHAO Tian-yu, JIAO Xin-yue, LIN Chun-jing, GUAN Zhe-yun, DING Xiao-yang, SUN Yan-yan, ZHANG Chun-bao. Comparative Transcriptome Analysis of Cytoplasmic Male Sterile Line and Its Restorer Line in Soybean [J]. Biotechnology Bulletin, 2024, 40(7): 137-149. |
| [5] | LIAO Yang-mei, ZHAO Guo-chun, WENG Xue-huang, JIA Li-ming, CHEN Zhong. Transcriptome Sequencing of Male Sterile Buds at Different Developmental Stages in Sapindus mukorossi ‘Qirui’ [J]. Biotechnology Bulletin, 2024, 40(7): 197-206. |
| [6] | BAI Zhi-yuan, XU Fei, YANG Wu, WANG Ming-gui, YANG Yu-hua, ZHANG Hai-ping, ZHANG Rui-jun. Transcriptome Analysis of Fertility Transformation in Weakly Restoring Hybrid F1 of Soybean Cytoplasmic Male Sterility [J]. Biotechnology Bulletin, 2024, 40(6): 134-142. |
| [7] | WU Di, YOU Xiao-feng, ZHENG Yi-zheng, LIN Nan, ZHANG Yan-yan, WEI Yi-cong. Analysis of Endogenous Hormone Regulation Mechanism for Carotenoid Synthesis in Sarcandra glabra [J]. Biotechnology Bulletin, 2024, 40(5): 203-214. |
| [8] | GUO Chun, SONG Gui-mei, YAN Yan, DI Peng, WANG Ying-ping. Genome Wide Identification and Expression Analysis of the bZIP Gene Family in Panax quinquefolius [J]. Biotechnology Bulletin, 2024, 40(4): 167-178. |
| [9] | ZHONG Yun, LIN Chun, LIU Zheng-jie, DONG Chen-wen-hua, MAO Zi-chao, LI Xing-yu. Cloning and Prokaryotic Expression Analysis of Asparagus Saponin Synthesis Related Glycosyltransferase Genes [J]. Biotechnology Bulletin, 2024, 40(4): 255-263. |
| [10] | YANG Qi, WEI Zi-di, SONG Juan, TONG Kun, YANG Liu, WANG Jia-han, LIU Hai-yan, LUAN Wei-jiang, MA Xuan. Construction and Transcriptomic Analysis of Rice Histone H1 Triple Mutant [J]. Biotechnology Bulletin, 2024, 40(4): 85-96. |
| [11] | XIE Qian, JIANG Lai, HE Jin, LIU Ling-ling, DING Ming-yue, CHEN Qing-xi. Regulatory Genes Mining Related to Transcriptome Sequencing and Phenolic Metabolism Pathway of Canarium album Fruit with Different Fresh Food Quality [J]. Biotechnology Bulletin, 2024, 40(3): 215-228. |
| [12] | LIANG Jia-lin, ZHAO Shuang, LI Xing-er, ZHAO Cheng-zhou, LI Ping. Identification of Aux/IAA Gene Family in Corydalis hendersonii Hemsl. and Analysis on Their Expression Pattern under UVB Treatment [J]. Biotechnology Bulletin, 2024, 40(12): 182-192. |
| [13] | LIU Shu-tong, WU Sheng, TAN Yi-yang, WANG De-pei, XUE Xian-li. Differential Analysis of Key Genes Involved in the Accumulation of L-malic Acid by Aspergillus niger Fermentation [J]. Biotechnology Bulletin, 2024, 40(12): 227-238. |
| [14] | LIANG Wan-feng, ZENG Jing-jing, HU Ruo-qun, CAO Jia-yu, ZHENG Tao, LI Luan, QIU Ming-yue, LIANG Xiao-ying, CHEN Ying. Transcriptional and Metabolomic Analysis of Carotenoid Accumulation in Anoectochilus roxburghii during Different Growth Periods [J]. Biotechnology Bulletin, 2024, 40(10): 262-274. |
| [15] | LI Ming-kun, BI Mei-ying, ZHANG Tian-hang, WU Xiang-yu, YANG Pei-ru, YING Ming. Restoration of Agricultural Function of Rhizobacteria by UgRNA/Cas9 Multi-gene Editing [J]. Biotechnology Bulletin, 2024, 40(10): 275-287. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||