








生物技术通报 ›› 2026, Vol. 42 ›› Issue (4): 190-201.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0966
杨婷( ), 杨宗桃, 艾静, 王禹童, 李燕烨, 邓军, 刘家勇, 赵勇(
), 杨宗桃, 艾静, 王禹童, 李燕烨, 邓军, 刘家勇, 赵勇( ), 张跃彬
), 张跃彬
收稿日期:2025-09-09
出版日期:2026-02-09
发布日期:2026-02-09
通讯作者:
赵勇,男,硕士,副研究员,研究方向 :甘蔗生理代谢和绿色生产技术;E-mail: 18087395132@163.com作者简介:杨婷,女,博士研究生,研究方向 :甘蔗分子育种;E-mail: 1146824026@qq.com
基金资助:
YANG Ting( ), YANG Zong-tao, AI Jing, WANG Yu-tong, LI Yan-ye, DENG jun, LIU Jia-yong, ZHAO Yong(
), YANG Zong-tao, AI Jing, WANG Yu-tong, LI Yan-ye, DENG jun, LIU Jia-yong, ZHAO Yong( ), ZHANG Yue-bin
), ZHANG Yue-bin
Received:2025-09-09
Published:2026-02-09
Online:2026-02-09
摘要:
目的 阐明甘蔗种质资源表型变异规律及其关键基因调控机制,为氮高效品种的定向选育提供分子靶点。 方法 以17份遗传背景清晰的甘蔗基因型材料为研究对象,系统测定株高、茎径、单茎重等7个农艺性状,评估其遗传多样性;通过聚类分析划分表型类群,并选取两类极端材料共6份进行根部转录组测序;采用DESeq2软件筛选差异表达基因(DEGs),并进行GO和KEGG功能富集分析;利用加权基因共表达网络分析(WGCNA)挖掘与类群分化相关的核心基因,并通过RT-qPCR验证其表达模式。此外,通过¹⁵N同位素吸收实验验证两类材料氮素吸收与利用能力的差异。 结果 17份材料表型变异系数为0.10-0.84,遗传多样性指数为2.55-2.83;聚类分析将其划分为Group Ι(7份)和Group Ⅱ(10份),两类群在茎径(1.12 cm vs 1.39 cm)、有效茎数(28.0 vs 15.1)及锤度(11.1 vs 14.9)等性状上差异明显。转录组分析共鉴定到9 724个DEGs,显著富集于亚油酸代谢(ko00591)、类黄酮生物合成(ko00941)、α-亚麻酸代谢(ko00592)、谷胱甘肽代谢(ko00480)、糖酵解/糖异生(ko00010)、乙醛酸与二羧酸代谢(ko00630)、淀粉和蔗糖代谢(ko00500)以及醚脂代谢(ko00565)等通路;WGCNA分析鉴定了10个与表型显著相关的枢纽基因,包括Sspon.02G0013210-1A(AP2转录因子)、Sspon.02G0008140-1T(GTP结合蛋白)等。¹⁵N吸收实验表明,Group II材料具有较强的氮素吸收能力,而Group I材料则表现出更高的氮积累特性。 结论 甘蔗野生种材料通过提高有效茎数适应低氮环境,而杂交种材料则通过增强氮吸收与利用效率实现生物量及糖分积累。关键基因协同调控碳氮代谢与氧化还原平衡是甘蔗氮效率形成的重要分子基础。
杨婷, 杨宗桃, 艾静, 王禹童, 李燕烨, 邓军, 刘家勇, 赵勇, 张跃彬. 不同基因型甘蔗表型特征及根部转录组学分析[J]. 生物技术通报, 2026, 42(4): 190-201.
YANG Ting, YANG Zong-tao, AI Jing, WANG Yu-tong, LI Yan-ye, DENG jun, LIU Jia-yong, ZHAO Yong, ZHANG Yue-bin. Analysis of Phenotypic Characteristics and Root Transcriptomics of Sugarcane with Different Genotypes[J]. Biotechnology Bulletin, 2026, 42(4): 190-201.
材料序号 Material number | 材料编码 Material code | 材料类型 Material type | 亲本信息(母本×父本) Parental cross (female × male) |
|---|---|---|---|
| 01 | 越南2号 | 野生种 | - |
| 02 | 云南82-1 | 野生种 | - |
| 03 | 2017-12-165 | 野生种 | - |
| 04 | 2017-22 | 野生种 | - |
| 05 | 云蔗0551 | 商品种 | - |
| 06 | A1 | 杂交种 | 云南82-1×2017-22 |
| 07 | A2 | 杂交种 | 云南82-1×2017-22 |
| 08 | B1 | 杂交种 | 越南2号×2017-12-165 |
| 09 | B2 | 杂交种 | 越南2号×2017-12-165 |
| 10 | N1 | 杂交种 | 云蔗0551×2017-22 |
| 11 | N2 | 杂交种 | 云蔗0551×越南2号 |
| 12 | N3 | 杂交种 | 云蔗0551×A1 |
| 13 | N4 | 杂交种 | 云蔗0551×B1 |
| 14 | N5 | 杂交种 | 云蔗0551×AB6 |
| 15 | N6 | 杂交种 | 云蔗0551×AB8 |
| 16 | AB6 | 杂交种 | A1×B1 |
| 17 | AB8 | 杂交种 | A1×B1 |
表1 17份甘蔗材料的遗传背景
Table 1 Genetic backgrounds of 17 sugarcane materials
材料序号 Material number | 材料编码 Material code | 材料类型 Material type | 亲本信息(母本×父本) Parental cross (female × male) |
|---|---|---|---|
| 01 | 越南2号 | 野生种 | - |
| 02 | 云南82-1 | 野生种 | - |
| 03 | 2017-12-165 | 野生种 | - |
| 04 | 2017-22 | 野生种 | - |
| 05 | 云蔗0551 | 商品种 | - |
| 06 | A1 | 杂交种 | 云南82-1×2017-22 |
| 07 | A2 | 杂交种 | 云南82-1×2017-22 |
| 08 | B1 | 杂交种 | 越南2号×2017-12-165 |
| 09 | B2 | 杂交种 | 越南2号×2017-12-165 |
| 10 | N1 | 杂交种 | 云蔗0551×2017-22 |
| 11 | N2 | 杂交种 | 云蔗0551×越南2号 |
| 12 | N3 | 杂交种 | 云蔗0551×A1 |
| 13 | N4 | 杂交种 | 云蔗0551×B1 |
| 14 | N5 | 杂交种 | 云蔗0551×AB6 |
| 15 | N6 | 杂交种 | 云蔗0551×AB8 |
| 16 | AB6 | 杂交种 | A1×B1 |
| 17 | AB8 | 杂交种 | A1×B1 |
基因ID/内参名称 Gene ID/Internal reference name | 正向引物 Forward primer (5′‒3′) | 反向引物 Reverse primer (5′‒3′) |
|---|---|---|
| Sspon.02G0008140-1T | GCCCATGGTGGTGGAGAC | CAACAGCCACCGTTTGCC |
| Sspon.05G0002890-3D | TTACCAGTGGGCTTGCGG | GCAAGTGCAGCCACATCG |
| Sspon.02G0013210-1A | ACTCGTCACAGCCAACCG | CCCGTCGCGTCGAATACA |
| Sspon.07G0022280-2C | GCCAGCGACTCTGCATCT | TGCTGCTGTGCAAAAGGC |
| Sspon.04G0031010-1P | GAGTGCCCTGTGTCGCAT | GTCTGGCCGCTGAACCTT |
| Sspon.01G0014700-2B | GGCTCCGAGGTTGCAGTT | TCTGAGCTTCTGCCCCCA |
| 25S rRNA1 | CCTGAAGATCACCCTGTGCT | GCAGTCTCCAGCTCCTGTTC |
表2 实时荧光定量PCR引物
Table 2 Primers used for RT-qPCR
基因ID/内参名称 Gene ID/Internal reference name | 正向引物 Forward primer (5′‒3′) | 反向引物 Reverse primer (5′‒3′) |
|---|---|---|
| Sspon.02G0008140-1T | GCCCATGGTGGTGGAGAC | CAACAGCCACCGTTTGCC |
| Sspon.05G0002890-3D | TTACCAGTGGGCTTGCGG | GCAAGTGCAGCCACATCG |
| Sspon.02G0013210-1A | ACTCGTCACAGCCAACCG | CCCGTCGCGTCGAATACA |
| Sspon.07G0022280-2C | GCCAGCGACTCTGCATCT | TGCTGCTGTGCAAAAGGC |
| Sspon.04G0031010-1P | GAGTGCCCTGTGTCGCAT | GTCTGGCCGCTGAACCTT |
| Sspon.01G0014700-2B | GGCTCCGAGGTTGCAGTT | TCTGAGCTTCTGCCCCCA |
| 25S rRNA1 | CCTGAAGATCACCCTGTGCT | GCAGTCTCCAGCTCCTGTTC |
性状 Trait | 最小值 Min | 最大值 Max | 平均值 Average | 极差 Range | 标准差 S | 变异系数 CV | 遗传多样性指数 H′ |
|---|---|---|---|---|---|---|---|
| 株高 PH (cm) | 218.00 | 314.60 | 253.61 | 96.60 | 24.67 | 0.10 | 2.83 |
| 茎径 SD (cm) | 0.74 | 2.51 | 1.28 | 1.77 | 0.48 | 0.37 | 2.77 |
| 茎节数 SNN (Nodes/m) | 16.40 | 31.00 | 24.05 | 14.60 | 3.59 | 0.15 | 2.82 |
| 节间长度 STL (cm) | 18.10 | 29.84 | 22.86 | 11.74 | 3.20 | 0.14 | 2.82 |
| 单茎重 SNW (kg) | 0.09 | 1.26 | 0.36 | 1.17 | 0.31 | 0.84 | 2.55 |
| 有效茎数 ESN (Stem nodes/plant) | 5.80 | 44.80 | 20.40 | 39.00 | 12.34 | 0.61 | 2.66 |
表3 17份甘蔗材料7个农艺性状的基本统计分析
Table 3 Basic statistical analysis of 7 agronomic traits of 17 sugarcane materials
性状 Trait | 最小值 Min | 最大值 Max | 平均值 Average | 极差 Range | 标准差 S | 变异系数 CV | 遗传多样性指数 H′ |
|---|---|---|---|---|---|---|---|
| 株高 PH (cm) | 218.00 | 314.60 | 253.61 | 96.60 | 24.67 | 0.10 | 2.83 |
| 茎径 SD (cm) | 0.74 | 2.51 | 1.28 | 1.77 | 0.48 | 0.37 | 2.77 |
| 茎节数 SNN (Nodes/m) | 16.40 | 31.00 | 24.05 | 14.60 | 3.59 | 0.15 | 2.82 |
| 节间长度 STL (cm) | 18.10 | 29.84 | 22.86 | 11.74 | 3.20 | 0.14 | 2.82 |
| 单茎重 SNW (kg) | 0.09 | 1.26 | 0.36 | 1.17 | 0.31 | 0.84 | 2.55 |
| 有效茎数 ESN (Stem nodes/plant) | 5.80 | 44.80 | 20.40 | 39.00 | 12.34 | 0.61 | 2.66 |
性状 Trait | 项目 Item | 种质类群 Germplasm population | ||
|---|---|---|---|---|
| Group Ι | Group Ⅱ | |||
| 株高PH (cm) | 平均值 Average | 252.57 | 254.34 | |
| 变异系数 CV | 0.09 | 0.11 | ||
| 平均茎径ASD (cm) | 平均值 Average | 1.12 | 1.39 | |
| 变异系数 CV | 0.56 | 0.24 | ||
| 茎节数SNN (Nodes/m) | 平均值 Average | 21.91 | 25.54 | |
| 变异系数 CV | 0.18 | 0.10 | ||
| 节间长度STL (cm) | 平均值 Average | 22.87 | 22.85 | |
| 变异系数 CV | 0.15 | 0.14 | ||
| 单茎重SNW (kg) | 平均值 Average | 0.31 | 0.40 | |
| 变异系数 CV | 1.39 | 0.51 | ||
| 有效茎数ESN(Stem nodes/plant) | 平均值 Average | 28.00 | 15.08 | |
| 变异系数 CV | 0.42 | 0.67 | ||
| 锤度B | 平均值 Average | 11.10 | 14.94 | |
| 变异系数 CV | 0.41 | 0.33 | ||
表4 两类群甘蔗材料农艺性状比较
Table 4 Comparison of agronomic traits between two groups of sugarcane materials
性状 Trait | 项目 Item | 种质类群 Germplasm population | ||
|---|---|---|---|---|
| Group Ι | Group Ⅱ | |||
| 株高PH (cm) | 平均值 Average | 252.57 | 254.34 | |
| 变异系数 CV | 0.09 | 0.11 | ||
| 平均茎径ASD (cm) | 平均值 Average | 1.12 | 1.39 | |
| 变异系数 CV | 0.56 | 0.24 | ||
| 茎节数SNN (Nodes/m) | 平均值 Average | 21.91 | 25.54 | |
| 变异系数 CV | 0.18 | 0.10 | ||
| 节间长度STL (cm) | 平均值 Average | 22.87 | 22.85 | |
| 变异系数 CV | 0.15 | 0.14 | ||
| 单茎重SNW (kg) | 平均值 Average | 0.31 | 0.40 | |
| 变异系数 CV | 1.39 | 0.51 | ||
| 有效茎数ESN(Stem nodes/plant) | 平均值 Average | 28.00 | 15.08 | |
| 变异系数 CV | 0.42 | 0.67 | ||
| 锤度B | 平均值 Average | 11.10 | 14.94 | |
| 变异系数 CV | 0.41 | 0.33 | ||
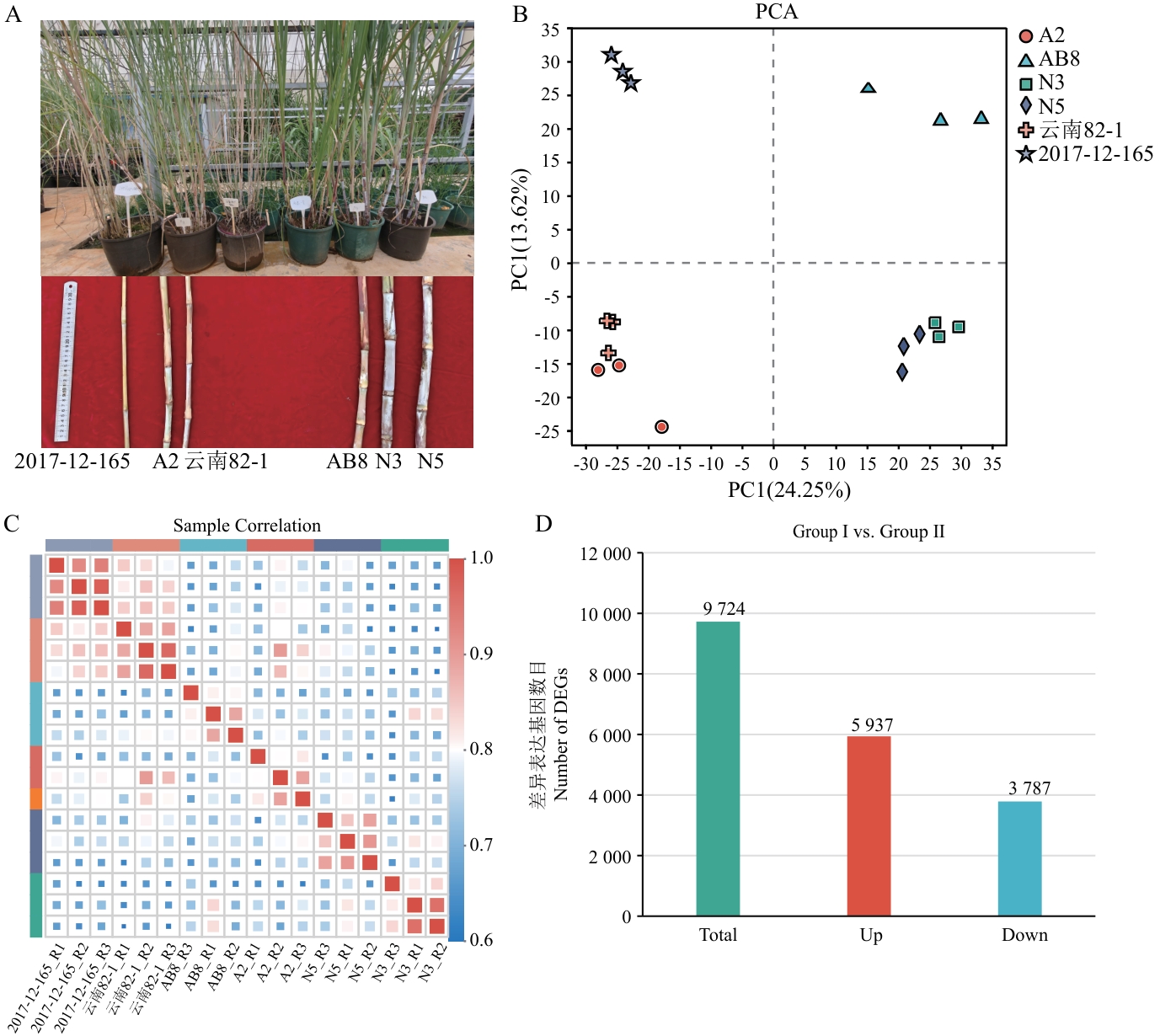
图2 转录组测序分析A:两类群6份材料的表型;B:PCA分析;C:样本间相关性分析;D:Group Ι vsGroup Ⅱ的差异表达基因柱状图
Fig. 2 Transcriptome sequencing analysisA: Phenotypes of six materials in two groups. B: PCA analysis. C: Correlation analysis among samples. D: Bar chart of DEGs between Group Ι and Group Ⅱ
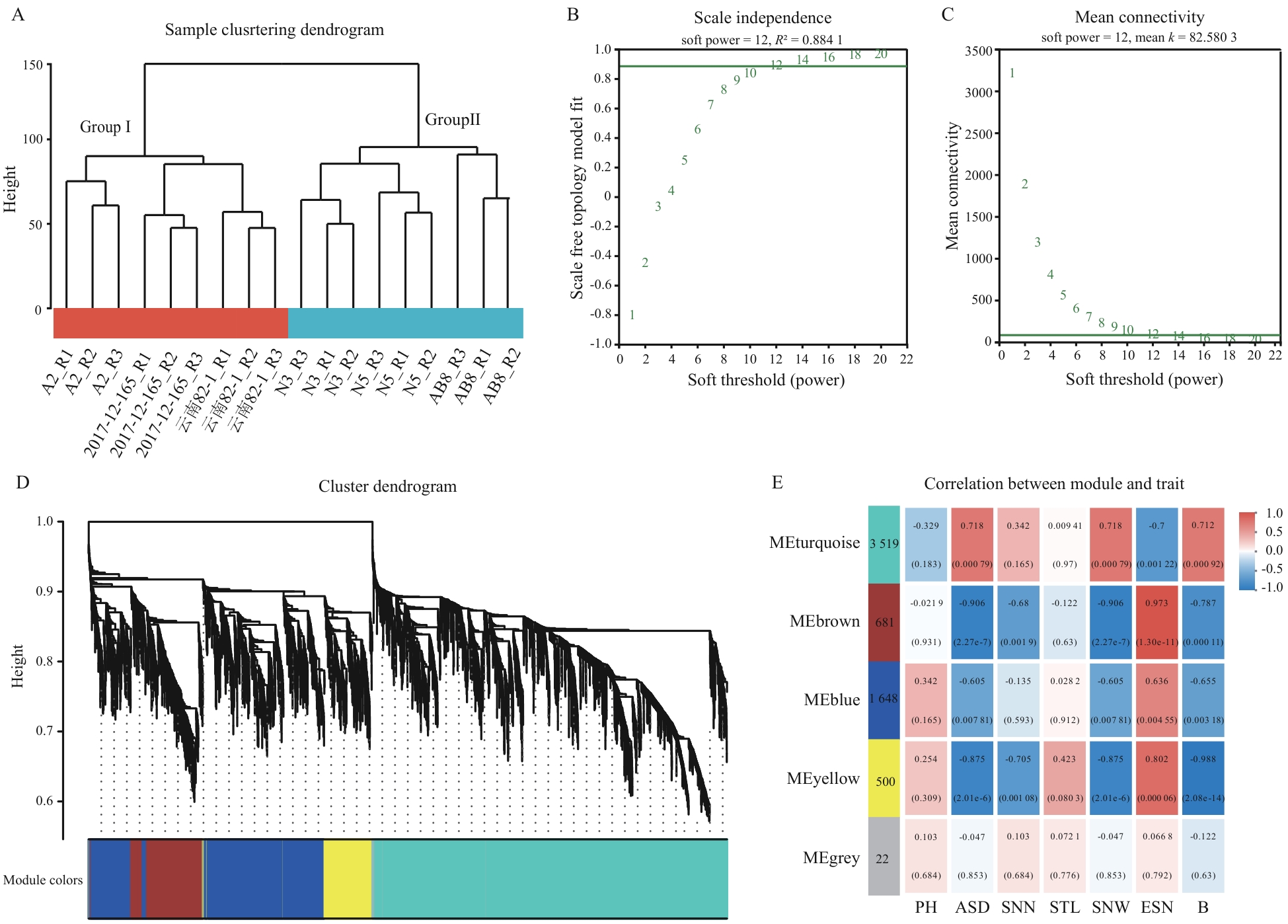
图4 加权基因共表达网络构建A:样本聚类;B:无尺度容适曲线;C:平均连通度曲线;D:基因聚类和模块构建;E:模块与表型相关性热图(红色格子代表样本与模块具有正相关性,蓝色格子代表样本与模块具有负相关性,图中括号内、外的数值分别为P值和相关系数r)
Fig. 4 Construction of WGCNAA: Sample clustering. B: Scale-free fit curve and average connectivity curve. C: Gene clustering and module construction. D: Heatmap of the correlation between modules and phenotypes (red grids indicate positive correlations between samples and modules, and purple grids indicate negative correlations between samples and modules. The values inside and outside the parentheses in the figure are the P-value and the correlation coefficient r, respectively)
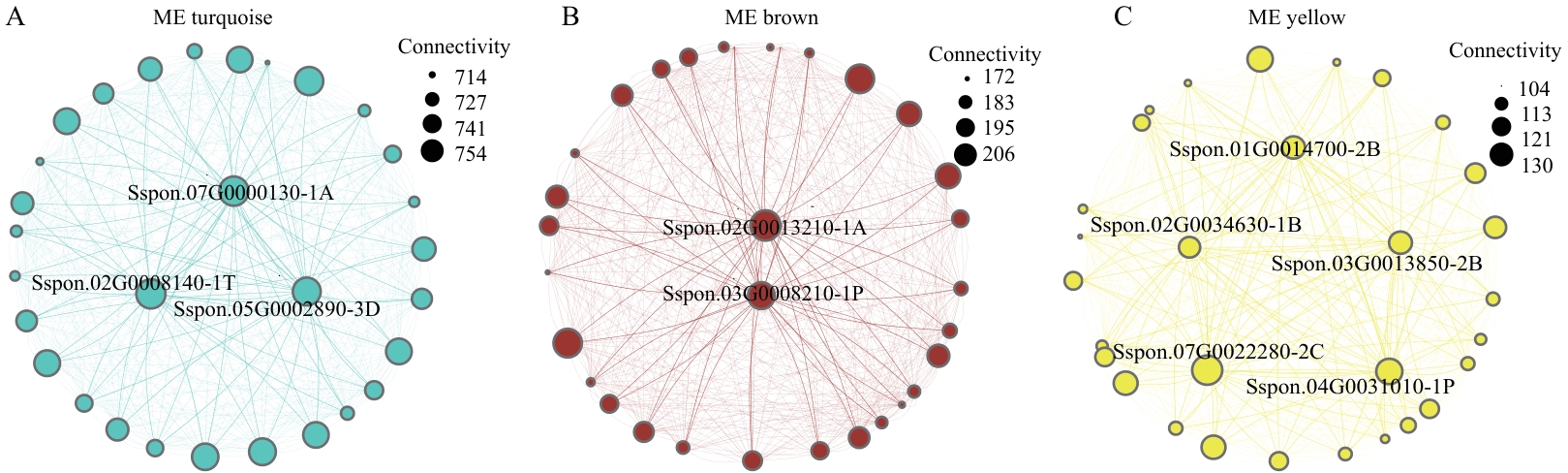
图5 关键模块内枢纽基因的互作网络A:蓝绿色模块;B:棕色模块;C:黄色模块(节点和标签大小代表基因的连通度)
Fig. 5 Interaction networks of hub genes within key modulesA: Turquoise module. B: Brown module. C: Yellow module (The sizes of nodes and labels indicatethe connectivity of genes)
模块 Module | 基因号 Gene ID | 功能注释 Annotation | KEGG注释 KEGG pathway | 蛋白结构域 PFAMs |
|---|---|---|---|---|
蓝绿色 Turquoise | Sspon.07G0000130-1A | Protein kinase superfamily | MAPK signaling pathway | Pkinase |
| Sspon.02G0008140-1T | This protein promotes the GTP-dependent binding of aminoacyl-tRNA to the A-site of ribosomes during protein biosynthesis | RNA transport | GTP_EFTU | |
| Sspon.05G0002890-3D | SIT4 phosphatase-associated protein | - | SAPS | |
棕色 Brown | Sspon.02G0013210-1A | DNA-binding domain in plant proteins such as APETALA2 and EREBPs | - | AP2 |
| Sspon.03G0008210-1P | Protein kinase domain | MAPK signaling pathway | Pkinase | |
黄色 Yellow | Sspon.07G0022280-2C | Belongs to the syntaxin family | SNARE interactions in vesicular transport | Syntaxin |
| Sspon.04G0031010-1P | Phosphotransferase enzyme family | Phosphotransferase enzyme family | ||
| Sspon.01G0014700-2B | Acyl transferase domain | Fatty acid biosynthesis | Acyl_transf_1 | |
| Sspon.03G0013850-2B | Dienelactone hydrolase family | - | NUDIX | |
| Sspon.02G0034630-1B | Belongs to the glycosyl hydrolase 17 family | Starch and sucrose metabolism | Glyco_hydro_17 |
表5 WGCNA筛选的候选枢纽基因及其注释
Table 5 Candidate hub genes identified by WGCNA and their functional annotations
模块 Module | 基因号 Gene ID | 功能注释 Annotation | KEGG注释 KEGG pathway | 蛋白结构域 PFAMs |
|---|---|---|---|---|
蓝绿色 Turquoise | Sspon.07G0000130-1A | Protein kinase superfamily | MAPK signaling pathway | Pkinase |
| Sspon.02G0008140-1T | This protein promotes the GTP-dependent binding of aminoacyl-tRNA to the A-site of ribosomes during protein biosynthesis | RNA transport | GTP_EFTU | |
| Sspon.05G0002890-3D | SIT4 phosphatase-associated protein | - | SAPS | |
棕色 Brown | Sspon.02G0013210-1A | DNA-binding domain in plant proteins such as APETALA2 and EREBPs | - | AP2 |
| Sspon.03G0008210-1P | Protein kinase domain | MAPK signaling pathway | Pkinase | |
黄色 Yellow | Sspon.07G0022280-2C | Belongs to the syntaxin family | SNARE interactions in vesicular transport | Syntaxin |
| Sspon.04G0031010-1P | Phosphotransferase enzyme family | Phosphotransferase enzyme family | ||
| Sspon.01G0014700-2B | Acyl transferase domain | Fatty acid biosynthesis | Acyl_transf_1 | |
| Sspon.03G0013850-2B | Dienelactone hydrolase family | - | NUDIX | |
| Sspon.02G0034630-1B | Belongs to the glycosyl hydrolase 17 family | Starch and sucrose metabolism | Glyco_hydro_17 |
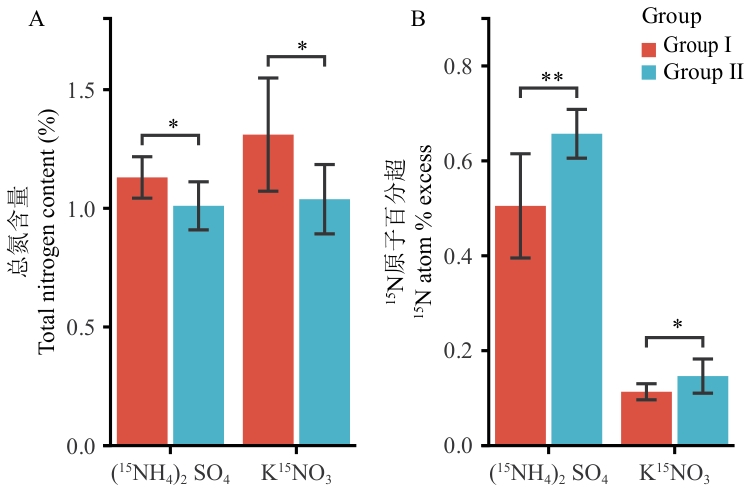
图7 两类群甘蔗根系在铵态氮与硝态氮下的总氮含量(A)及¹⁵N吸收(B)动力学数据表示为平均值±标准差(n = 3)。采用独立样本t检验进行组间差异显著性分析。*和**分别表示在Group I和Group II在同一氮源处理下差异显著(* P < 0.05;** P < 0.01)
Fig. 7 Dynamics of root total nitrogen content (A) and ¹⁵N uptake (B) under ammonium and nitrate nutrition in two sugarcane groupsData are presented as mean ± SD (n = 3). Significant differences between Group I and Group II under the same nitrogen source were determined by independent-samples t test. * and ** indicate significant differences at P < 0.05 and P < 0.01, respectively
| [1] | Islam MS, Yang XP, Sood S, et al. Molecular characterization of genetic basis of Sugarcane yellow leaf virus (SCYLV) resistance in Saccharum spp. hybrid [J]. Plant Breed, 2018, 137(4): 598-604. |
| [2] | Sharma A, Chandra A. Identification of new Leuconostoc species responsible for post-harvest sucrose losses in sugarcane [J]. Sugar Tech, 2018, 20(4): 492-496. |
| [3] | Goldemberg J. The Brazilian biofuels industry [J]. Biotechnol Biofuels, 2008, 1(1): 6. |
| [4] | Frink CR, Waggoner PE, Ausubel JH. Nitrogen fertilizer: retrospect and prospect [J]. Proc Natl Acad Sci USA, 1999, 96(4): 1175-1180. |
| [5] | Garnett T, Conn V, Kaiser BN. Root based approaches to improving nitrogen use efficiency in plants [J]. Plant Cell Environ, 2009, 32(9): 1272-1283. |
| [6] | 翁梦静, 张慈凤, 林娜平, 等. 甘蔗ScmiR393在植物应答缺氮胁迫中的功能研究 [J]. 福建农业科技, 2025, 56(1): 24-30. |
| Weng MJ, Zhang CF, Lin NP, et al. Functional study of sugarcane ScmiR393 in plant response to nitrogen deficiency stress [J]. Fujian Agric Sci Technol, 2025, 56(1): 24-30. | |
| [7] | Robinson N, Brackin R, Vinall K, et al. Nitrate paradigm does not hold up for sugarcane [J]. PLoS One, 2011, 6(4): e19045. |
| [8] | 李文宝. 甘蔗营养高效利用种质资源筛选研究 [D]. 南宁: 广西大学, 2011. |
| Li WB. Screenning for higher nutrients use efficiency of sugarcane germplasm [D]. Nanning: Guangxi University, 2011. | |
| [9] | 杨荣仲, 周会, 桂意云, 等. 甘蔗低氮胁迫性状变化与蔗茎含氮量的研究 [J]. 安徽农业科学, 2013, 41(2): 481-484. |
| Yang RZ, Zhou H, Gui YY, et al. Response to low nitrogen stress and stalk nitrogen content of sugarcane germplasm [J]. J Anhui Agric Sci, 2013, 41(2): 481-484. | |
| [10] | Fan XR, Feng HM, Tan YW, et al. A putative 6-transmembrane nitrate transporter OsNRT1.1b plays a key role in rice under low nitrogen [J]. J Integr Plant Biol, 2016, 58(6): 590-599. |
| [11] | Ge M, Wang YC, Liu YH, et al. The NIN-like protein 5 (ZmNLP5) transcription factor is involved in modulating the nitrogen response in maize [J]. Plant J, 2020, 102(2): 353-368. |
| [12] | 杨文亭, 李志贤, 赖健宁, 等. 甘蔗‒大豆间作和减量施氮对甘蔗产量和主要农艺性状的影响 [J]. 作物学报, 2014, 40(3): 556-562. |
| Yang WT, Li ZX, Lai JN, et al. Effects of sugarcane-soybean intercropping and reduced nitrogen application on yield and major agronomic traits of sugarcane [J]. Acta Agron Sin, 2014, 40(3): 556-562. | |
| [13] | de Castro SGQ, Magalhães PSG, de Castro SAQ, et al. Optimizing nitrogen fertilizer rates at distinct in-season application moments in sugarcane [J]. Int J Plant Prod, 2022, 16(1): 137-152. |
| [14] | 张艳梅. 施氮水平对不同甘蔗品种产量、品质及生理生化特性的影响 [D]. 南宁: 广西大学, 2015. |
| Zhang YM. Effects of nitrogen levels on cane yield and quality, and physiological and biochemical characteristics of different sugarcane varieties [D]. Nanning: Guangxi University, 2015. | |
| [15] | Huang DL, Qin CX, Gui YY, et al. Role of the SPS gene families in the regulation of sucrose accumulation in sugarcane [J]. Sugar Tech, 2017, 19(2): 117-124. |
| [16] | Verma AK, Upadhyay SK, Verma PC, et al. Functional analysis of sucrose phosphate synthase (SPS) and sucrose synthase (SS) in sugarcane (Saccharum) cultivars [J]. Plant Biol, 2011, 13(2): 325-332. |
| [17] | 陈俊华, 文吉富, 王国良, 等. Excel在计算群落生物多样性指数中的应用 [J]. 四川林业科技, 2009, 30(3): 88-90, 60. |
| Chen JH, Wen JF, Wang GL, et al. On application of excel in calculating the biodiversity index of communities [J]. J Sichuan For Sci Technol, 2009, 30(3): 88-90, 60. | |
| [18] | 李旭娟, 李纯佳, 刘洪博, 等. 甘蔗腋芽形成发育过程的转录组分析 [J]. 生物技术通报, 2025, 41(3): 202-218. |
| Li XJ, Li CJ, Liu HB, et al. Transcriptome analysis of axillary bud formation and development in sugarcane [J]. Biotechnol Bull, 2025, 41(3): 202-218. | |
| [19] | 阙友雄, 许莉萍, 徐景升, 等. 甘蔗基因表达定量PCR分析中内参基因的选择 [J]. 热带作物学报, 2009, 30(3): 274-278. |
| Que YX, Xu LP, Xu JS, et al. Selection of control genes in real-time qPCR analysis of gene expression in sugarcane [J]. Chin J Trop Crops, 2009, 30(3): 274-278. | |
| [20] | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method [J]. Methods, 2001, 25(4): 402-408. |
| [21] | 李俊杰, 杜蒲芳, 石婷瑞, 等. 不同基因型小麦苗期耐低氮性评价及筛选 [J]. 中国农业科技导报, 2021, 23(7): 21-32. |
| Li JJ, Du PF, Shi TR, et al. Screening and evaluation of low nitrogen tolerance from different genotypes wheat at seedling stage [J]. J Agric Sci Technol, 2021, 23(7): 21-32. | |
| [22] | Liu YQ, Wang HR, Jiang ZM, et al. Genomic basis of geographical adaptation to soil nitrogen in rice [J]. Nature, 2021, 590(7847): 600-605. |
| [23] | Zhan J, Zhou YF, Yang LS, et al. Low ammonium and high nitrate input improves nitrogen use efficiency and growth in sugarcane through coordinated reprogramming of nitrogen and carbon metabolism [J]. Plant Physiol Biochem, 2025, 229: 110354. |
| [24] | 江厚龙, 李勇, 陈天才, 等. 综合转录组和代谢组分析揭示嫁接提高烟草钾利用效率的分子机制 [J]. 天津农业科学, 2025, 31(4): 1-12, 19. |
| Jiang HL, Li Y, Chen TC, et al. Integrated transcriptomic and metabolomic analyses elucidate the mechanism underlying the impact of grafting on potassium utilization efficiency in tobacco [J]. Tianjin Agric Sci, 2025, 31(4): 1-12, 19. | |
| [25] | 李寒雪. 代谢组和转录组联合解析藜麦幼苗对氮素的响应机制 [D]. 昆明: 云南农业大学, 2024. |
| Li HX. Transcriptome and metabolome analyses revealed the response mechanism of quinoa seedlings to nitrogen fertilizer [D]. Kunming: Yunnan Agricultural University, 2024. | |
| [26] | 南运有. BnaA5.AIB与BnaC 2.NAR2.1参与甘蓝型油菜对外源脱落酸的应答响应及氮吸收利用的调控 [D]. 杨凌: 西北农林科技大学, 2023. |
| Nan YY. BnaA5.AIB and BnaC 2.NAR2.1 are involved in the response to exogenous abscisic acid and the regulation of nitrogen uptake and utilization in Brassica napus L. [D]. Yangling: Northwest A & F University, 2023. | |
| [27] | 葛礼姣. 氮高效利用菊花品种筛选及高效利用机理研究 [D]. 南京: 南京农业大学, 2022. |
| Ge LJ. Study on nitrogen efficient variety screening and its mechanism on high nitrogen utilization efficiency in Chrysanthemum [D]. Nanjing: Nanjing Agricultural University, 2022. | |
| [28] | 任盼荣, 汪军成, 姚立蓉, 等. 大麦种质资源磷利用效率的评价及其转录组分析 [J]. 大麦与谷类科学, 2018, 35(4): 56-57. |
| Ren PR, Wang JC, Yao LR, et al. Evaluation of phosphorus use efficiency and transcriptome analysis in barley germplasm (Hordeum vulgare L.) [J]. Barley Cereal Sci, 2018, 35(4): 56-57. | |
| [29] | Liu H, Gao XH, Fan WS, et al. Optimizing carbon and nitrogen metabolism in plants: From fundamental principles to practical applications [J]. J Integr Plant Biol, 2025, 67(6): 1447-1466. |
| [30] | 刘潇潇. 膜脂在小麦低氮胁迫响应中的作用及机制研究 [D]. 北京: 中国科学院大学(中国科学院教育部水土保持与生态环境研究中心), 2021. |
| Liu XX. The mechanism of the involvement of leaf membrane lipids in response to nitrogen deficiency in wheat [D]. Beijing: Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, 2021. | |
| [31] | 梁桂红, 华营鹏, 周婷, 等. 甘蓝型油菜NRT1.5和NRT1.8家族基因的生物信息学分析及其对氮-镉胁迫的响应 [J]. 作物学报, 2019, 45(3): 365-380. |
| Liang GH, Hua YP, Zhou T, et al. Bioinformatics analysis and response to nitrate-cadmium stress of NRT1.5 and NRT1.8 family genes in Brassica napus [J]. Acta Agron Sin, 2019, 45(3): 365-380. | |
| [32] | 钱甫, 张占琴, 陈树宾, 等. 基于GWAS和WGCNA分析挖掘玉米花期相关候选基因 [J]. 作物学报, 2023, 49(12): 3261-3276. |
| Qian F, Zhang ZQ, Chen SB, et al. Mining maize flowering traits related candidate genes based on GWAS and WGCNA data [J]. Acta Agron Sin, 2023, 49(12): 3261-3276. | |
| [33] | 郝瑞杰, 邱晨, 耿晓云, 等. 梅花PmABCG9在苯甲醇挥发中的功能分析 [J]. 中国农业科学, 2023, 56(13): 2574-2585. |
| Hao RJ, Qiu C, Geng XY, et al. The function of PmABCG9 transporter related to the volatilization of benzyl alcohol in Prunus mume [J]. Sci Agric Sin, 2023, 56(13): 2574-2585. | |
| [34] | 荐红举, 张梅花, 尚丽娜, 等. 利用WGCNA筛选马铃薯块茎发育候选基因 [J]. 作物学报, 2022, 48(7): 1658-1668. |
| Jian HJ, Zhang MH, Shang LN, et al. Screening candidate genes involved in potato tuber development using WGCNA [J]. Acta Agron Sin, 2022, 48(7): 1658-1668. | |
| [35] | 张恒燕, 张木清. 基于WGCNA挖掘外源GA3调节甘蔗节间伸长相关基因 [J/OL]. 分子植物育种, 2022. . |
| Zhang HY, Zhang MQ. Mining of genes related to exogenous GA3 regulation of sugarcane internode elongation based on WGCNA [J/OL]. Mol Plant Breed, 2022. . | |
| [36] | Wang L, Ming LC, Liao KY, et al. Bract suppression regulated by the miR156/529-SPLs-NL1-PLA1 module is required for the transition from vegetative to reproductive branching in rice [J]. Mol Plant, 2021, 14(7): 1168-1184. |
| [37] | 汪宽鸿, 祝彪, 朱祝军. GSH/GSSG在植物应对非生物胁迫中的作用综述 [J]. 园艺学报, 2021, 48(4): 647-660. |
| Wang KH, Zhu B, Zhu ZJ. Review of the role of GSH/GSSG in plant abiotic stress response [J]. Acta Hortic Sin, 2021, 48(4): 647-660. | |
| [38] | 祁雪姣, 谢乾坤, 谢雨欣, 等. 籼稻品种Y两优886高氮素利用效率机制研究 [J]. 河南农业科学, 2021, 50(3): 25-32. |
| Qi XJ, Xie QK, Xie YX, et al. Mechanism of high nitrogen utilization efficiency of indica rice yliangyou 886 [J]. J Henan Agric Sci, 2021, 50(3): 25-32. | |
| [39] | 吕江艳, 龙鹏宇, 罗维钢, 等. 甘蔗节水高产和蔗田氧化亚氮减排的滴灌施肥模式 [J]. 节水灌溉, 2023(12): 1-8. |
| Lü JY, Long PY, Luo WG, et al. Drip irrigation fertilization model for water-saving and high-yield sugarcane and reduction of nitrous oxide emissions in sugarcane fields [J]. Water Sav Irrig, 2023(12): 1-8. |
| [1] | 王玉昆, 原远, 王斌, 朱云娜, 任晓强, 任飞, 叶红. 转录组和脂质代谢组联合分析不同紫苏α-亚麻酸合成调控差异[J]. 生物技术通报, 2026, 42(4): 129-140. |
| [2] | 刘青媛, 吴洪启, 陈秀娥, 陈剑, 姜远泽, 何燕子, 喻奇伟, 刘仁祥. 转录因子NtMYB96a调控烟草耐旱性的功能研究[J]. 生物技术通报, 2026, 42(4): 239-250. |
| [3] | 樊荣辉, 罗远华, 陈艺荃, 方能炎, 陈燕, 钟淮钦, 叶秀仙. 文心兰‘金辉’和‘香水文心’花香形成比较分析[J]. 生物技术通报, 2026, 42(1): 105-113. |
| [4] | 刘建国, 刘格儿, 郭颖欣, 王斌, 王玉昆, 卢金凤, 黄文庭, 朱云娜. 转录组和代谢组联合解析‘桂柚1号’和‘沙田柚’果实品质差异[J]. 生物技术通报, 2025, 41(9): 168-181. |
| [5] | 刘泽洲, 段乃彬, 岳丽昕, 王清华, 姚行浩, 高莉敏, 孔素萍. 大蒜叶片蜡质成分分析及蜡质缺失基因Ggl-1筛选[J]. 生物技术通报, 2025, 41(9): 219-231. |
| [6] | 闫梦阳, 梁晓阳, 戴君昂, 张妍, 关团, 张辉, 刘良波, 孙志华. 阿莫西林降解菌的筛选及降解机制研究[J]. 生物技术通报, 2025, 41(9): 314-325. |
| [7] | 柴军发, 洪波, 贾彦霞. 转录组和代谢组联合分析三株蜡蚧轮枝菌菌株毒力差异[J]. 生物技术通报, 2025, 41(8): 311-321. |
| [8] | 白雨果, 李婉迪, 梁建萍, 石志勇, 卢庚龙, 刘红军, 牛景萍. 哈茨木霉T9131对黄芪幼苗的促生机理[J]. 生物技术通报, 2025, 41(8): 175-185. |
| [9] | 王月琛, 韩鑫骐, 魏文敏, 崔兆兰, 罗阳美, 陈鹏如, 王海岗, 刘龙龙, 张莉, 王纶. 黍稷落粒的生物学基础研究及落粒调控基因的鉴定[J]. 生物技术通报, 2025, 41(7): 164-171. |
| [10] | 张津浩, 邓辉, 张清壮, 陶禹, 周池, 李鑫. 贝莱斯芽胞杆菌XY40-1对百合球茎生长、品质及镉含量的调控作用[J]. 生物技术通报, 2025, 41(7): 281-291. |
| [11] | 张越, 毕钰, 慕雪男, 郑子薇, 王志刚, 徐伟慧. 小麦赤霉病拮抗菌JB7的生防特性[J]. 生物技术通报, 2025, 41(7): 261-271. |
| [12] | 董栩焜, 车永梅, 王明硕, 罗政刚, 管恩森, 赵方贵, 叶青, 刘新. 纳米硅和蜡样芽胞杆菌SS1共处理对烟草生长的影响[J]. 生物技术通报, 2025, 41(7): 292-298. |
| [13] | 李成花, 豆飞飞, 任毓昭, 刘彩霞, 刘凤楼, 王掌军, 李清峰. 外施水杨酸对白粉菌侵染小麦的影响及白粉菌转录组分析[J]. 生物技术通报, 2025, 41(7): 272-280. |
| [14] | 郭秀娟, 冯宇, 吴瑞香, 王利琴, 杨建春. Ca2+处理对胡麻种子萌发影响的转录组分析[J]. 生物技术通报, 2025, 41(7): 139-149. |
| [15] | 胡若群, 曾菁菁, 梁婉凤, 曹佳玉, 黄小苇, 梁晓英, 仇明月, 陈莹. 转录组和代谢组联合分析探究不同遮光条件下金线莲类胡萝卜素合成代谢机制[J]. 生物技术通报, 2025, 41(5): 231-243. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||