








生物技术通报 ›› 2025, Vol. 41 ›› Issue (4): 123-133.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0877
• 研究报告 • 上一篇
李文兰1( ), 侯辛未1, 李燕2, 赵瑞君2, 孟昭东1, 岳润清1(
), 侯辛未1, 李燕2, 赵瑞君2, 孟昭东1, 岳润清1( )
)
收稿日期:2024-09-09
出版日期:2025-04-26
发布日期:2025-04-25
通讯作者:
岳润清,女,博士,研究员,研究方向 :作物遗传育种;E-mail: yuerunqing@126.com作者简介:李文兰,女,博士,助理研究员,研究方向 :分子遗传育种;E-mail: liwenlantutu@126.com
基金资助:
LI Wen-lan1( ), HOU Xin-wei1, LI Yan2, ZHAO Rui-jun2, MENG Zhao-dong1, YUE Run-qing1(
), HOU Xin-wei1, LI Yan2, ZHAO Rui-jun2, MENG Zhao-dong1, YUE Run-qing1( )
)
Received:2024-09-09
Published:2025-04-26
Online:2025-04-25
摘要:
目的 在回交转育过程中利用分子检测手段将转基因玉米LD05目标性状准确快速导入育种常规自交系中,并明确LD05纯杂合株系和对照郑58在抗虫融合基因m2cryAb-vip3A表达水平、抗虫性和农艺性状等方面是否存在差异。 方法 通过左右边界引物进行PCR扩增鉴定外源目的基因m2cryAb-vip3A在自交系中的纯杂合,利用RT-qPCR和ELISA开展m2cryAb-vip3A在转录和翻译水平的表达分析,并通过室内生测和田间接虫试验鉴定LD05纯杂合株系对靶标害虫亚洲玉米螟、草地贪夜蛾、黏虫和棉铃虫的抗性,通过田间调查和室内考种对LD05纯杂合株系和对照郑58在农艺性状方面的差异进行比较分析。 结果 通过筛选优化鉴定,确定LC915+LC966为最优纯杂合鉴定引物。纯杂合株系中外源插入基因m2cryAb-vip3A在转录和翻译水平存在差异,纯合株系中普遍高于杂合株系。室内生测结果显示,喂食LD05纯合株系和杂合株系心叶期叶片,玉米螟、草地贪夜蛾、黏虫的校正死亡率均达到100%,为高抗级别;田间接虫试验结果显示,LD05纯合株系和杂合株系在心叶期和花丝期对玉米螟、心叶期对黏虫、花丝期对棉铃虫的抗性等级均为高抗。农艺性状调查显示LD05纯合株系、杂合株系和对照郑58无差别。 结论 建立了基于普通PCR的LD05转化体中目的基因的纯杂合鉴定方法,明确外源目的基因m2cryAb-vip3A在LD05纯杂合株系中表达存在差异,但在抗虫性和农艺性状等方面没有显著差异。
李文兰, 侯辛未, 李燕, 赵瑞君, 孟昭东, 岳润清. 转基因抗虫耐除草剂玉米LD05纯杂合植株的鉴定及抗性检测[J]. 生物技术通报, 2025, 41(4): 123-133.
LI Wen-lan, HOU Xin-wei, LI Yan, ZHAO Rui-jun, MENG Zhao-dong, YUE Run-qing. Identification and Resistance Detection of Homozygous and Heterozygous Plants of Transgenic Maize LD05 with Resistances to Insect and Herbicide[J]. Biotechnology Bulletin, 2025, 41(4): 123-133.
| 引物名称 Primer name | 引物序列 Primer sequence (5'-3') | 引物名称 Primer name | 引物序列 Primer sequence (5'-3') |
|---|---|---|---|
| LC913 | AAGTGTTCAGTAAATTATAT | LC953 | TGGCGTTACCCAACTTAATC |
| LC915 | ATACGCCTGTCAAGTGTCAT | LC958 | AATTGGTTCTTGGAATCGCA |
| LC917 | CCTTGTCCTGCATTGCGCAT | LC962 | ATATGTTTCTCAGCGAGCAT |
| LC937 | TCTACTTGGCAAAGGCTTCAGAT | LC964 | CACGGTTCCAGAGGAAAACC |
| LC947 | CAGTAAATTATATACGCCTG | LC966 | GTGCTAGTCAATAGAACTA |
| LC936 | GCACCATCGTCAACCACTACA | LC968 | CACATCGCTAGCTAGTGCTA |
| LC938 | TCTGGCAGCTGGACTTCAGCCTG | LC928 | CGCATAGAAACAACAGAAGTG |
| LC944 | CCAGTACTAAAATCCAGATC | LC929 | TCCTAAAACCAAAATCCAGTA |
| LC948 | CGTCCGCAATGTGTTATTAA | LC933 | GGCAGAGGCATCTTCAACG |
| LC931 | CTTGATGAGACCTGCTGCGT | LC934 | AAACCCCAAGTCCAAGTAAC |
| LC941 | TGAGACCTGCTGCGTAAGCC | zSSIIb-F | CTCCCAATCCTTTGACATCTGC |
| LC951 | AGCTTGGCACTGGCCGTCG | zSSIIb-R | TCGATTTCTCTCTTGGTGACAGG |
表1 设计引物的序列信息
Table 1 Sequence information for designing primers
| 引物名称 Primer name | 引物序列 Primer sequence (5'-3') | 引物名称 Primer name | 引物序列 Primer sequence (5'-3') |
|---|---|---|---|
| LC913 | AAGTGTTCAGTAAATTATAT | LC953 | TGGCGTTACCCAACTTAATC |
| LC915 | ATACGCCTGTCAAGTGTCAT | LC958 | AATTGGTTCTTGGAATCGCA |
| LC917 | CCTTGTCCTGCATTGCGCAT | LC962 | ATATGTTTCTCAGCGAGCAT |
| LC937 | TCTACTTGGCAAAGGCTTCAGAT | LC964 | CACGGTTCCAGAGGAAAACC |
| LC947 | CAGTAAATTATATACGCCTG | LC966 | GTGCTAGTCAATAGAACTA |
| LC936 | GCACCATCGTCAACCACTACA | LC968 | CACATCGCTAGCTAGTGCTA |
| LC938 | TCTGGCAGCTGGACTTCAGCCTG | LC928 | CGCATAGAAACAACAGAAGTG |
| LC944 | CCAGTACTAAAATCCAGATC | LC929 | TCCTAAAACCAAAATCCAGTA |
| LC948 | CGTCCGCAATGTGTTATTAA | LC933 | GGCAGAGGCATCTTCAACG |
| LC931 | CTTGATGAGACCTGCTGCGT | LC934 | AAACCCCAAGTCCAAGTAAC |
| LC941 | TGAGACCTGCTGCGTAAGCC | zSSIIb-F | CTCCCAATCCTTTGACATCTGC |
| LC951 | AGCTTGGCACTGGCCGTCG | zSSIIb-R | TCGATTTCTCTCTTGGTGACAGG |
| 引物名称 Primer name | 引物序列 Primer sequence (5'-3') | 引物名称 Primer name | 引物序列 Primer sequence (5'-3') |
|---|---|---|---|
| m2cryAb-vip3A-F | GTTTCCTTTACCGGGGACGA | 18S-F | AAACGGCTACCACATCCAAG |
| m2cryAb-vip3A-A | ACCACCCCCTTCAACTTCAG | 18S-A | CCTCCAATGGATCCTCGTTA |
表2 设计定量引物的序列信息
Table 2 Sequence information of the designed RT-qPCR primers
| 引物名称 Primer name | 引物序列 Primer sequence (5'-3') | 引物名称 Primer name | 引物序列 Primer sequence (5'-3') |
|---|---|---|---|
| m2cryAb-vip3A-F | GTTTCCTTTACCGGGGACGA | 18S-F | AAACGGCTACCACATCCAAG |
| m2cryAb-vip3A-A | ACCACCCCCTTCAACTTCAG | 18S-A | CCTCCAATGGATCCTCGTTA |
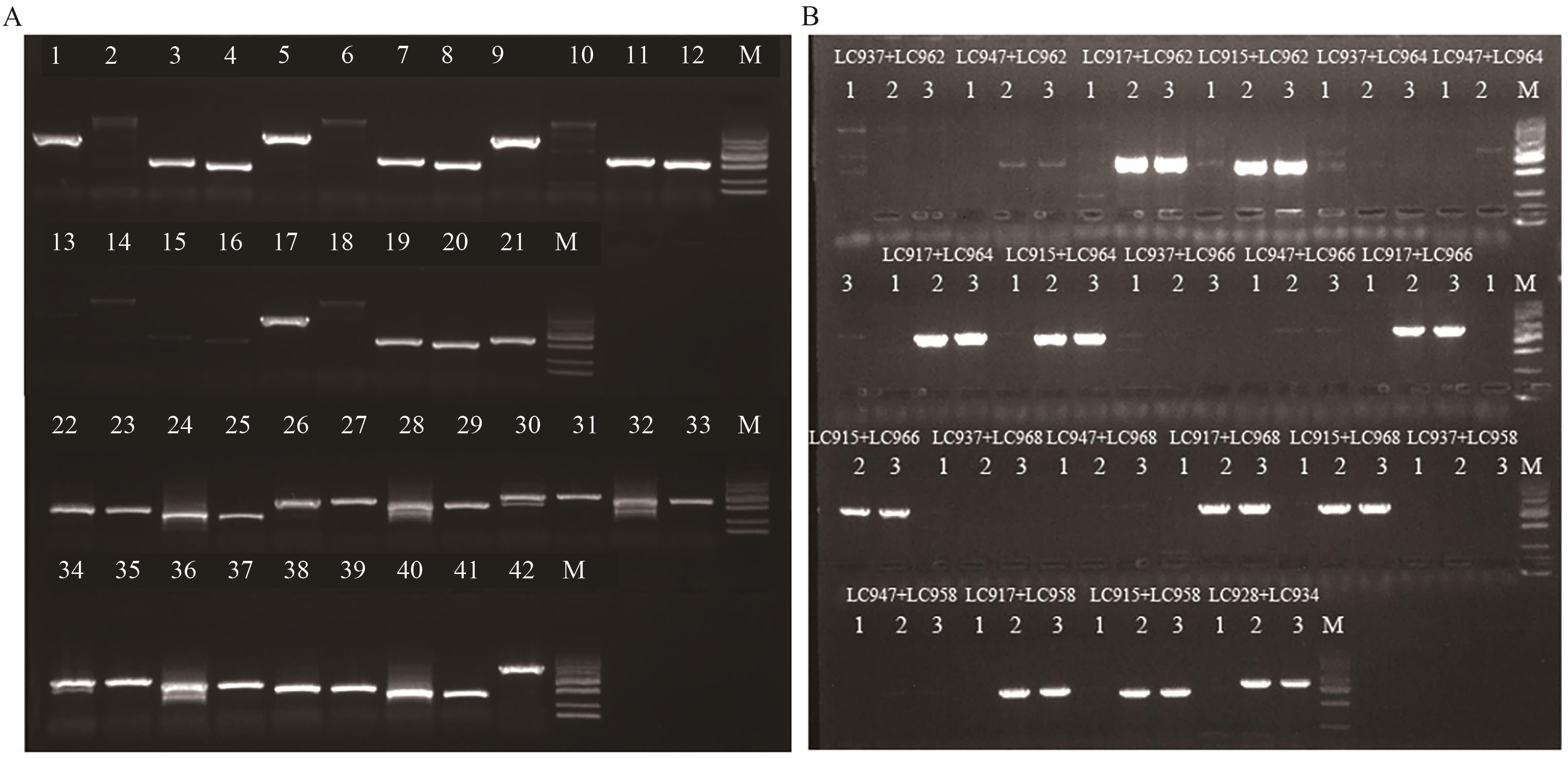
图2 LD05中外源目的基因的纯杂合鉴定引物筛选A:LD05转化体特异性PCR引物筛选;M:DL2000 Maker;1:引物LC937和LC936的扩增产物;2:引物LC937和LC938的扩增产物;3:引物LC937和LC944的扩增产物;4:引物LC937和LC948的扩增产物;5:引物LC947和LC936的扩增产物;6:引物LC947和LC938的扩增产物;7:引物LC947和LC944的扩增产物;8:引物LC947和LC948的扩增产物;9:引物LC917和LC936的扩增产物;10:引物LC917和LC938的扩增产物;11:引物LC917和LC944的扩增产物;12:引物LC917和LC948的扩增产物;13:引物LC913和LC936的扩增产物;14:引物LC913和LC938的扩增产物;15:引物LC913和LC944的扩增产物;16:引物LC913和LC948的扩增产物;17:引物LC915和LC936的扩增产物;18:引物LC915和LC938的扩增产物;19:引物LC915和LC944的扩增产物;20:引物LC915和LC948的扩增产物;21:引物LC928和LC929的扩增产物;22:引物LC931和LC962的扩增产物;23:引物LC941和LC962的扩增产物;24:引物LC951和LC962的扩增产物;25:引物LC953和LC962的扩增产物;26:引物LC931和LC964的扩增产物;27:引物LC941和LC964的扩增产物;28:引物LC951和LC964的扩增产物;29:引物LC953和LC964的扩增产物;30:引物LC931和LC966的扩增产物;31:引物LC941和LC966的扩增产物;32:引物LC951和LC966的扩增产物;33:引物LC953和LC966的扩增产物;34:引物LC931和LC968的扩增产物;35:引物LC941和LC968的扩增产物;36:引物LC951和LC968的扩增产物;37:引物LC953和LC968的扩增产物;38:引物LC931和LC958的扩增产物;38:引物LC941和LC958的扩增产物;40:引物LC951和LC958的扩增产物;41:引物LC953和LC958的扩增产物;42:引物LC933和LC934的扩增产物。B:LD05中外源目的基因的纯杂合鉴定引物筛选;1:纯合DNA样本;2:杂合DNA样本;3:野生型DNA样本;M:DL2000 marker,条带从上往下依次代表2 000、1 000、750、500、250、100 bp
Fig. 2 Screening of primers for homozygous and heterozygous identification of target gene in LD05A: Screening of specific PCR primers for LD05 transformants. M: DL2000 maker. 1: Amplified products of primers LC937 and LC936. 2: Amplified products of primers LC937 and LC938. 3: Amplified products of primers LC937 and LC944. 4: Amplified products of primers LC937 and LC948. 5: Amplified products of primers LC947 and LC936. 6: Amplified products of primers LC947 and LC938. 7: Amplified products of primers LC947 and LC944. 8: Amplified products of primers LC947 and LC948. 9: Amplified products of primers LC917 and LC936. 10: Amplified products of primers LC917 and LC938. 11: Amplified products of primers LC917 and LC944. 12: Amplified products of primers LC917 and LC948. 13: Amplified products of primers LC913 and LC936. 14: Amplified products of primers LC913 and LC938. 15: Amplified products of primers LC913 and LC944. 16: Amplified products of primers LC913 and LC948. 17: Amplified products of primers LC915 and LC936. 18: Amplified products of primers LC915 and LC938. 19: Amplified products of primers LC915 and LC944. 20: Amplified products of primers LC915 and LC948. 21: LC928 and LC929. 22: Amplified products of primers LC931 and LC962. 23: Amplified products of primers LC941 and LC962. 24: Amplified products of primers LC951 and LC962. 25: Amplified products of primers LC953 and LC962. 26: Amplified products of primers LC931 and LC964. 27: Amplified products of primers LC941 and LC964. 28: Amplified products of primers LC951 and LC964. 29: Amplified products of primers LC953 and LC964. 30: Amplified products of primers LC931 and LC966. 31: Amplified products of primers LC941 and LC966. 32: Amplified products of primers LC951 and LC966. 33: Amplified products of primers LC953 and LC966. 34: Amplified products of primers LC931 and LC968. 35: Amplified products of primers LC941 and LC968. 36: Amplified products of primers LC951 and LC968. 37: Amplified products of primers LC953 and LC968. 38: Amplified products of primers LC931 and LC958. 38: Amplified products of primers LC941 and LC958. 40: Amplified products of primers LC951 and LC958. 41: Amplified products of primers LC953 and LC958. 42: Amplified products of primers LC933 and LC934. B: Screening of primers for homozygous and heterozygous identification of target gene in LD05; 1: homozygous DNA sample; 2: heterozygous DNA sample; 3: wild-type DNA sample; M: DL2000 marker, the bands from top to bottom represent 2 000, 1 000, 750, 500, 250, and 100 bp

图3 不同引物组合的扩增循环数对扩增效率的影响和检出限测试A:不同引物组合的扩增循环数对扩增效率的影响;1、7、13、19、25、31:循环数20;2、8、14、20、26、32:循环数22;3、9、15、21、27、33:循环数24;4、10、16、22、28、34:循环数26;5、11、17、23、29、35:循环数28;6、12、18、24、30、36:循环数30;M:DL2000 marker,条带从上往下依次代表2 000、1 000、750、500、250、100 bp。B:不同引物组合的检出限测试;1、7、13、19、25、31:模板浓度为100 ng/µL;2、8、14、20、26、32:模板浓度为10 ng/µL;3、9、15、21、27、33:模板浓度为1 ng/µL;4、10、16、22、28、34:模板浓度为0.1 ng/µL;5、11、17、23、29、35:模板浓度为0.01 ng/µL;6、12、18、24、30、36:模板浓度为0.001 ng/µL;M:DL2000 marker
Fig. 3 Effects of different amplification cycles on amplification efficiency and detection limit test of different primer combinationsA: Effect of different amplification cycles on amplification efficiency; 1, 7, 13, 19, 25, 31: 20 cycles; 2, 8, 14, 20, 26, 32: 22 cycles; 3, 9, 15, 21, 27, 33: 24 cycles; 4, 10, 16, 22, 28, 34: 26 cycles; 5, 11, 17, 23, 29, 35: 28 cycles; 6, 12, 18, 24, 30, 36: 30 cycles; M: DL2000 marker, the bands from top to bottom represent 2 000, 1 000, 750, 500, 250, 100 bp. B: Detection limit test of different primer combinations; 1, 7, 13, 19, 25, 31: template concentration is 100 ng/µL; 2, 8, 14, 20, 26, 32: template concentration of 10 ng/µL; 3, 9, 15, 21, 27, 33: the template concentration is 1 ng/µL; 4, 10, 16, 22, 28, 34: the template concentration is 0.1 ng/µL; 5, 11, 17, 23, 29, 35: the template concentration is 0.01 ng/µL; 6, 12, 18, 24, 30, 36: template concentration is 0.001 ng/µL; M: DL2000 marker

图4 纯杂合引物测试分析A:引物LC915+LC966的PCR扩增结果;B:引物LC915+LC948的PCR扩增结果。1-93:BC6F2植株的93个随机DNA样本;94:空白对照;95:阴性对照;96:阳性对照;M:DL2000 marker,条带从上往下依次代表2 000、1 000、750、500、250、100 bp
Fig. 4 Analysis of homozygous and heterozygous primersA: PCR amplified results of primer LC915+LC966; B: PCR amplified results of primer LC915+LC948. 1-93: 93 random DNA samples from BC6F2 plants; 94: blank control; 95: negative control; 96: positive control; M: DL2000 marker, the bands from top to bottom represent 2 000, 1 000, 750, 500, 250, and 100 bp

图5 m2cryAb-vip3A在纯杂合株系中的RT-qPCR和ELISA分析A:m2cryAb-vip3A在纯杂合株系中的RT-qPCR分析;B:m2cryAb-vip3A编码蛋白在纯杂合株系中的ELISA分析。1、5、8、20:杂合株系;3、7、17:纯合株系。柱形条上的字母(或字母数字组合)代表利用邓肯多重检测分析表达差异显著性(P<0.05)
Fig. 5 RT-qPCR and ELISA analysis of m2cryAb-vip3A in homozygous and heterozygous plantsA: RT-qPCR analysis of m2cryAb-vip3A in homozygous and heterozygous plants; B: ELISA analysis of m2cryAb-vip3A encoded protein in homozygous and heterozygous plants. 1, 5, 8, 20: heterozygous plants; 3, 7, 17: homozygous plants. The letters (or alphanumeric combinations) in bars indicate the significant difference in expression analysis by Duncan multiple detection(P<0.05)
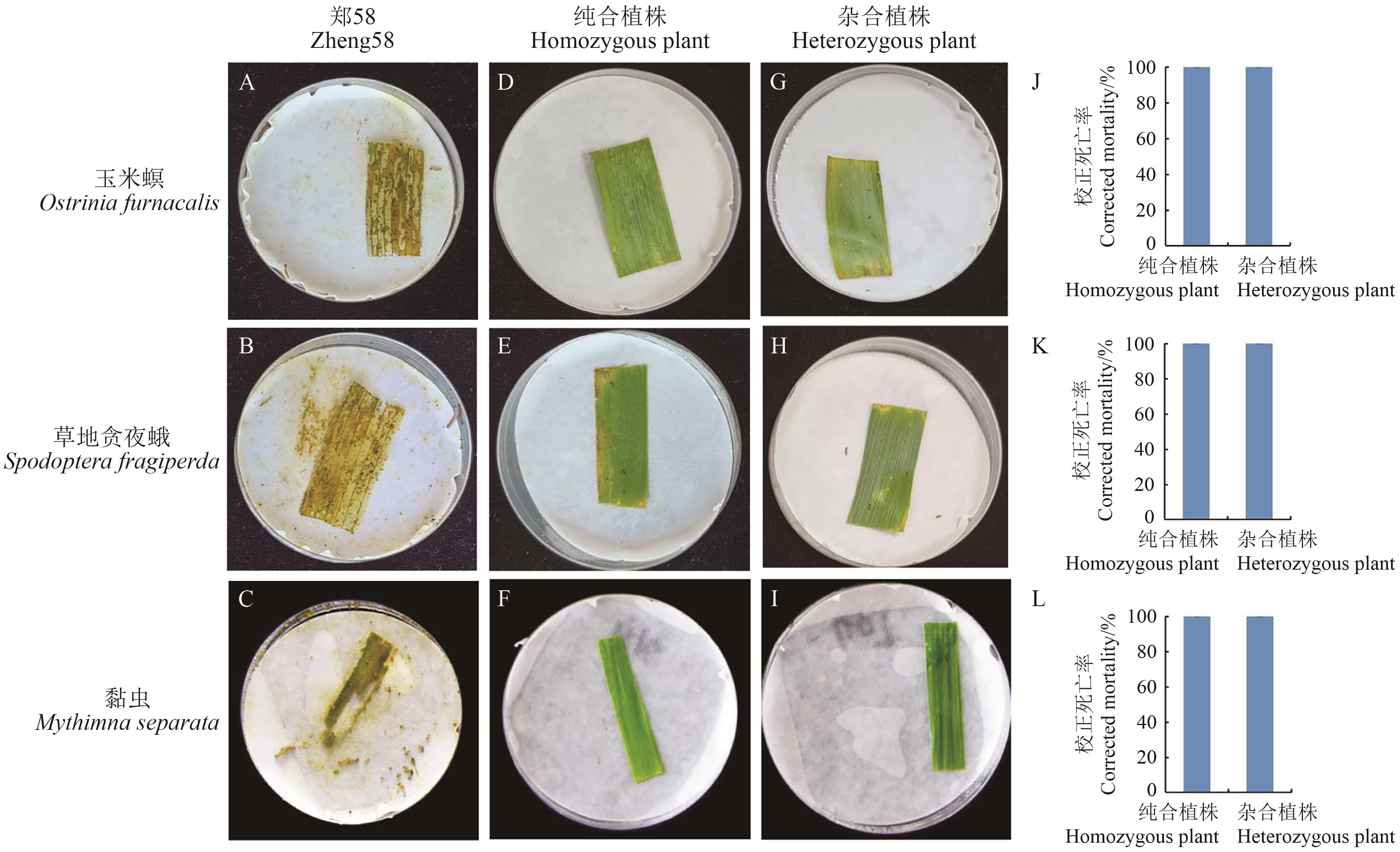
图6 玉米心叶期叶片对玉米螟、草地贪夜蛾和黏虫的室内生测结果(接虫后6 d)A-C:郑58心叶期叶片;D-F:纯合植株心叶期叶片;G-I:杂合植株心叶期叶片。A、D、G:试虫为玉米螟;B、E、H:试虫为草地贪夜蛾;C、F、I:试虫为黏虫;J:玉米螟校正死亡率;K:草地贪夜蛾校正死亡率;L:黏虫校正死亡率
Fig. 6 Results of laboratory bioassay on Ostrinia furnacalis, Spodoptera fragiperda and Mythimna separata at the heart leaf stage (6 d after inoculation)A-C: Zheng 58 leaves at V5 stage; D-F: leaves of homozygous plants at V5 stage; G-I: leaves of heterozygous plants atV5 stage.A, D, G: Ostrinia furnacalis; B, E, H: Spodoptera fragiperda; C, F, I: Mythimna separata; J: corrected mortality of Ostrinia furnacalis; K: corrected mortality of Spodoptera fragiperda; L: corrected mortality of Mythimna separata
靶标害虫 Target pests | 接种时期 Inoculation period | 纯合植株 Homozygous plant | 杂合植株 Heterozygous plant | 郑58 Zheng 58 | ||||
|---|---|---|---|---|---|---|---|---|
级值 Level value | 抗性 Resistance | 级值 Level value | 抗性 Resistance | 级值 Level value | 抗性 Resistance | |||
玉米螟 Ostrinia furnacalis | 心叶期 V5 stage | 1.0 | 高抗 High resistance | 1.0 | 高抗 High resistance | 8.90 | 高感 High sense | |
花丝期 Silking stage | 1.0 | 高抗 High resistance | 1.0 | 高抗 High resistance | 8.99 | 高感 High sense | ||
黏虫 Mythimna separata | 心叶期 V5 stage | 1.01 | 高抗 High resistance | 1.02 | 高抗 High resistance | 8.65 | 高感 High sense | |
棉铃虫 Helicoverpa armigera | 花丝期 Silking stage | 1.0 | 高抗 High resistance | 1.0 | 高抗 High resistance | 6.85 | 感 Sense | |
表3 玉米螟、黏虫和棉铃虫田间接虫结果分析
Table 3 Analysis field trials of Ostrinia furnacalis, Helicoverpa armigera and Helicoverpa armigera
靶标害虫 Target pests | 接种时期 Inoculation period | 纯合植株 Homozygous plant | 杂合植株 Heterozygous plant | 郑58 Zheng 58 | ||||
|---|---|---|---|---|---|---|---|---|
级值 Level value | 抗性 Resistance | 级值 Level value | 抗性 Resistance | 级值 Level value | 抗性 Resistance | |||
玉米螟 Ostrinia furnacalis | 心叶期 V5 stage | 1.0 | 高抗 High resistance | 1.0 | 高抗 High resistance | 8.90 | 高感 High sense | |
花丝期 Silking stage | 1.0 | 高抗 High resistance | 1.0 | 高抗 High resistance | 8.99 | 高感 High sense | ||
黏虫 Mythimna separata | 心叶期 V5 stage | 1.01 | 高抗 High resistance | 1.02 | 高抗 High resistance | 8.65 | 高感 High sense | |
棉铃虫 Helicoverpa armigera | 花丝期 Silking stage | 1.0 | 高抗 High resistance | 1.0 | 高抗 High resistance | 6.85 | 感 Sense | |
| 农艺性状 Agronomic trait | LD05纯合株系 Homozygous plant | 杂合株系 Heterozygous plant | 郑58 Zheng 58 |
|---|---|---|---|
| 抽雄期Tasseling stage/d | 59.7±1.2a | 59.0±1.0a | 59.3±0.6a |
| 生育期Growth stage/d | 109.0±1.0a | 109.3±0.6a | 109.7±1.2a |
| 株高Plant height/cm | 163.7±1.5a | 163.0±4.0a | 164.3±1.5a |
| 穗位高Ear height/cm | 56.3±2.1a | 56.7±0.6a | 56.0±1.0a |
| 穗行数Rows per ear | 12±0a | 12±0a | 12±0a |
| 穗粗Ear diameter/cm | 3.8±0.1a | 3.8±0.0a | 3.8±0.1a |
| 百粒重100-kernel weight/g | 33.2±0.9a | 33.0±0.2a | 33.6±0.7a |
表4 LD05纯合株系、杂合株系和对照郑58农艺性状比较分析
Table 4 Comparative analysis of agronomic characters between LD05 of homozygous and heterozygous plants and Zheng 58
| 农艺性状 Agronomic trait | LD05纯合株系 Homozygous plant | 杂合株系 Heterozygous plant | 郑58 Zheng 58 |
|---|---|---|---|
| 抽雄期Tasseling stage/d | 59.7±1.2a | 59.0±1.0a | 59.3±0.6a |
| 生育期Growth stage/d | 109.0±1.0a | 109.3±0.6a | 109.7±1.2a |
| 株高Plant height/cm | 163.7±1.5a | 163.0±4.0a | 164.3±1.5a |
| 穗位高Ear height/cm | 56.3±2.1a | 56.7±0.6a | 56.0±1.0a |
| 穗行数Rows per ear | 12±0a | 12±0a | 12±0a |
| 穗粗Ear diameter/cm | 3.8±0.1a | 3.8±0.0a | 3.8±0.1a |
| 百粒重100-kernel weight/g | 33.2±0.9a | 33.0±0.2a | 33.6±0.7a |
| 1 | 李浩辉, 刘彩月, 张海文, 等. 2022年度全球转基因作物产业化发展现状及趋势分析 [J]. 中国农业科技导报, 2023, 25(12): 6-16. |
| Li HH, Liu CY, Zhang HW, et al. Global genetically modified crop industrialization trends in 2022 [J]. J Agric Sci Technol, 2023, 25(12): 6-16. | |
| 2 | 李婷婷, 李文娟. 我国玉米空间格局演变及其影响因素研究进展 [J]. 中国农业资源与区划, 2021, 42(2): 87-95. |
| Li TT, Li WJ. Research progress on the evolition of maize spatial pattern and its influencing factors in China [J]. Chin J Agric Resour Reg Plan, 2021, 42(2): 87-95. | |
| 3 | Zhao SY, Yang XM, Liu DZ, et al. Performance of the domestic Bt corn event expressing pyramided Cry1Ab and Vip3Aa19 against the invasive Spodoptera frugiperda (J. E. Smith) in China [J]. Pest Manag Sci, 2023, 79(3): 1018-1029. |
| 4 | 张思雨, 林朝阳, 叶雨轩, 等. 转cry1Ab-vip3Af2和cp4-epsps基因的抗虫耐除草剂水稻的研究 [J]. 浙江农业学报, 2023, 35(8): 1823-1833. |
| Zhang SY, Lin CY, Ye YX, et al. Characterization of transgenic insect resistance and glyphosate tolerance rice expressing cry1Ab-vip3Af2 and cp4-epsps [J]. Acta Agric Zhejiangensis, 2023, 35(8): 1823-1833. | |
| 5 | 林海燕. 表达Cry-VIP3和CP4EPSPS蛋白的转基因抗虫耐草甘膦豇豆的研究 [D]. 杭州: 浙江大学, 2022. |
| Lin HY. A transgenic insect resistance and herbicide tolerance cowpea expression Cry-VIP3 and CP4EPSPS [D]. Hangzhou: Zhejiang University, 2022. | |
| 6 | 黎裕, 王天宇. 玉米转基因技术研发与应用现状及展望 [J]. 玉米科学, 2018, 26(2): 1-15, 22. |
| Li Y, Wang TY. Germplasm enhancement in maize: advances and prospects [J]. J Maize Sci, 2018, 26(2): 1-15, 22. | |
| 7 | 孙伟, 张江丽, 黄丛林, 等. 玉米遗传转化方法的研究进展 [J]. 安徽农业科学, 2007, 35(25): 7772-7774. |
| Sun W, Zhang JL, Huang CL, et al. Progresses in maize genetic transformation techniques [J]. J Anhui Agric Sci, 2007, 35(25): 7772-7774. | |
| 8 | 张元昶, 张首国, 李振科, 等. 转基因玉米遗传转化的研究进展 [J]. 重庆工商大学学报: 自然科学版, 2006, 23(5): 473-476, 495. |
| Zhang YC, Zhang SG, Li ZK, et al. Research progress in genetic transformation methods of transgenic maize [J]. J Chongqing Technol Bus Univ Nat Sci Ed, 2006, 23(5): 473-476, 495. | |
| 9 | Ishida Y, Hiei Y, Komari T. Agrobacterium-mediated transformation of maize [J]. Nat Protoc, 2007, 2(7): 1614-1621. |
| 10 | 周洪昌, 宋伟, 王凤格, 等. 玉米丝黑穗病分子标记辅助选择育种中前景引物与背景引物的筛选 [J]. 分子植物育种, 2011, 9(4): 450-456. |
| Zhou HC, Song W, Wang FG, et al. The selection of foreground and background markers used in maize resistance head smut marker assisted breeding [J]. Mol Plant Breed, 2011, 9(4): 450-456. | |
| 11 | German MA, Kandel- Kfir M, Swarzberg D, et al. A rapid method for the analysis of zygosity in transgenic plants [J]. Plant Science, 2003, 164(2): 183-187. |
| 12 | Sridevi G, Parameswari C, Rajamuni P, et al. Identification of hemizygous and homozygous transgenic rice plants in T1 generation by DNA blot analysis [J]. Plant Biotechnol, 2006, 23(5): 531-534. |
| 13 | 贾芝琪, 张忠华, 崔艳红, 等. 利用外源基因侧翼序列扩增筛选番茄纯合转基因植株 [J]. 农业生物技术学报, 2009, 17(5): 820-824. |
| Jia ZQ, Zhang ZH, Cui YH, et al. Screening homozygous transgenic plants by flanking sequences amplification of T-DNA in tomatoes [J]. J Agric Biotechnol, 2009, 17(5): 820-824. | |
| 14 | 张焕春, 汪小福, 李玥莹, 等. 转Cry1Ab水稻纯合体快速准确的PCR鉴定方法 [J]. 浙江农业学报, 2012, 24(4): 549-554. |
| Zhang HC, Wang XF, Li YY, et al. A rapid and accurate PCR method for homozygous lines screening for genetically modified rice containing Cry1Ab [J]. Acta Agric Zhejiangensis, 2012, 24(4): 549-554. | |
| 15 | 岳润清, 李文兰, 孟昭东. 转基因抗虫耐除草剂玉米自交系LG11的获得及抗性分析 [J]. 作物学报, 2024, 50(1): 89-99. |
| Yue RQ, Li WL, Meng ZD. Acquisition and resistance analysis of transgenic Maize Inbred Line LG11 with insect and herbicide resistance [J]. Acta Agron Sin, 2024, 50(1): 89-99. | |
| 16 | 王振营, 王晓鸣. 我国玉米病虫害发生现状、趋势与防控对策 [J]. 植物保护, 2019, 45(1): 1-11. |
| Wang ZY, Wang XM. Current status and management strategies for corn pests and diseases in China [J]. Plant Prot, 2019, 45(1): 1-11. | |
| 17 | 孙红炜, 李凡, 高瑞, 等. 转cry1Ab/cry2Aj和G10evo-epsps基因玉米中Bt蛋白的时空表达及抗性评价 [J]. 生物安全学报, 2018, 27(1): 63-68. |
| Sun HW, Li F, Gao R, et al. Bt protein spatial-temporal expression and evaluation for resistance of transgenic cry1Ab/cry2Aj and G10evo-epsps maize [J]. J Biosaf, 2018, 27(1): 63-68. | |
| 18 | 王振, 高树仁, 王霞, 等. 玉米不育系回交转育群体背景选择效果的研究 [J]. 玉米科学, 2018, 26(2): 23-29. |
| Wang Z, Gao SR, Wang X, et al. Effect of backcross breeding of maize male sterile line on population background selection [J]. J Maize Sci, 2018, 26(2): 23-29. | |
| 19 | 朱少喜, 金肇阳, 葛建镕, 等. 基于KASP平台的转基因玉米高通量特异性检测方法 [J]. 生物技术通报, 2023, 39(6): 133-140. |
| Zhu SX, Jin ZY, Ge JR, et al. High-throughput specific detection methods for transgenic maize based on the KASP platform [J]. Biotechnol Bull, 2023, 39(6): 133-140. | |
| 20 | 朱少喜, 张云龙, 赵怡锟, 等. 基于60K芯片的玉米回交群体精准背景选择策略 [J]. 玉米科学, 2023, 31(4): 43-51. |
| Zhu SX, Zhang YL, Zhao YK, et al. Accurate background selection strategy of maize backcross population based on 60K chip [J]. J Maize Sci, 2023, 31(4): 43-51. | |
| 21 | 石优. 分子标记辅助选择改良玉米自交系小斑病抗性 [D]. 杨凌: 西北农林科技大学, 2023. |
| Shi Y. Molecular marker-assisted selection for improving resistance of maize inbred lines to leaf spot disease [D]. Yangling: Northwest A & F University, 2023. | |
| 22 | Bilbo TR, Reay-Jones FPF, Reisig DD, et al. Development, survival, and feeding behavior of Helicoverpa zea (Lepidoptera: Noctuidae) relative to Bt protein concentrations in corn ear tissues [J]. PLoS One, 2019, 14(8): e0221343. |
| 23 | 李香菊. 我国耐除草剂转基因作物研发与产业化应用前景 [J]. 植物保护, 2023, 49(5): 316-324. |
| Li XJ. Development of herbicide tolerant crops and their commercialization in China [J]. Plant Prot, 2023, 49(5): 316-324. | |
| 24 | 龙丽坤, 赵宁, 夏蔚, 等. 转基因玉米CM8101特异性定性PCR检测方法 [J]. 中国农学通报, 2021, 37(23): 23-28. |
| Long LK, Zhao N, Xia W, et al. CM8101 transgenic maize: qualitative PCR assay detection of specificity [J]. Chin Agric Sci Bull, 2021, 37(23): 23-28. | |
| 25 | 李葱葱, 谢苹, 董立明, 等. 抗虫耐除草剂玉米GH5112E-117C定性PCR检测方法 [J]. 生物技术通报, 2020, 36(5): 64-67. |
| Li CC, Xie P, Dong LM, et al. Qualitative PCR assay for the detection of GH5112E-117C transgenic maize resistant to insects and herbicides [J]. Biotechnol Bull, 2020, 36(5): 64-67. | |
| 26 | 李凌燕, 肖冰, 张旭冬, 等. 转基因耐除草剂玉米MON87419品系特异性定性PCR检测方法的建立 [J]. 江苏农业科学, 2023, 51(1): 50-57. |
| Li LY, Xiao B, Zhang XD, et al. Establishment of specific qualitative PCR detection method for transgenic herbicide-tolerant maize strain MON87419 [J]. Jiangsu Agric Sci, 2023, 51(1): 50-57. | |
| 27 | 白耀宇, 蒋明星, 程家安. Bt水稻Cry1Ab杀虫蛋白表达的时间动态及其在水稻土中的降解 [J]. 生态学报, 2005, 25(7): 1583-1590. |
| Bai YY, Jiang MX, Cheng JA. Temporal expression patterns of Cry1Ab insecticidal protein in Bt rice plants and its degradation in paddy soils [J]. Acta Ecol Sin, 2005, 25(7): 1583-1590. | |
| 28 | 姜志磊, 刘德璞, 李晓辉, 等. 转基因抗虫玉米Bt毒蛋白的时空表达分析 [J]. 吉林农业科学, 2008, 33(6): 35-37. |
| Jiang ZL, Liu DP, Li XH, et al. Studies on the temporal and spatial expressions of bt toxin protein of bt transgenic maize [J]. J Jilin Agric Sci, 2008, 33(6): 35-37. | |
| 29 | Trtikova M, Wikmark OG, Zemp N, et al. Transgene expression and Bt protein content in transgenic Bt maize (MON810) under optimal and stressful environmental conditions [J]. PLoS One, 2015, 10(4): e0123011. |
| 30 | 王冬梅, 李海强, 丁瑞丰, 等. 新疆北部地区转Bt基因棉外源杀虫蛋白表达时空动态研究 [J]. 棉花学报, 2012, 24(1): 18-26. |
| Wang DM, Li HQ, Ding RF, et al. Spatio-temporal expression of foreign bt insecticidal protein in transgenic bt cotton varieties in northern Xinjiang Province, China [J]. Cotton Sci, 2012, 24(1): 18-26. |
| [1] | 刘彤彤, 李肖慧, 杨骏龙, 陈旺, 玉猛, 王超凡, 王凤茹, 客绍英. ZmSTART1调控玉米维管束建成的功能研究[J]. 生物技术通报, 2025, 41(4): 115-122. |
| [2] | 王涛, 胡社伟, 张宇, 邓文文, 尚春缘, 王婉艺. 玉米籽粒淀粉生物合成及调控因素研究进展[J]. 生物技术通报, 2025, 41(3): 1-13. |
| [3] | 任鑫茹, 赵宏璐, 李雅静, 刘荣军, 曾凡力, 王钦宏, 王震. 混菌低温发酵对玉米秸秆黄贮饲料品质的影响[J]. 生物技术通报, 2025, 41(3): 330-342. |
| [4] | 田栩瑞, 霍信屹, 郭云涵, 向林, 产祝龙, 王艳平. 百合LoSAUR10基因的表达特征及功能分析[J]. 生物技术通报, 2025, 41(1): 221-229. |
| [5] | 任晓敏, 云岚, 艾芊, 赵乔. 新麦草异戊烯基转移酶PjIPT基因的功能验证[J]. 生物技术通报, 2024, 40(7): 207-215. |
| [6] | 王秋月, 段鹏亮, 李海笑, 刘宁, 曹志艳, 董金皋. 玉米大斑病菌cDNA文库的构建及转录因子StMR1互作蛋白的筛选[J]. 生物技术通报, 2024, 40(6): 281-289. |
| [7] | 胡锦锦, 李素贞, 马旭辉, 柳小庆, 谢珊珊, 江海洋, 陈茹梅. 玉米花青素生物合成代谢调控[J]. 生物技术通报, 2024, 40(6): 34-44. |
| [8] | 孙亚楠, 王春雪, 王欣, 杜秉海, 刘凯, 汪城墙. 萎缩芽孢杆菌CNY01的生防特性及其对玉米的抗盐促生作用[J]. 生物技术通报, 2024, 40(5): 248-260. |
| [9] | 王佳玮, 李晨, 刘建利, 周世杰, 易嘉敏, 杨谨源, 康鹏. 内生真菌接种方式对青贮玉米幼苗生长的影响[J]. 生物技术通报, 2024, 40(4): 189-202. |
| [10] | 胡伊娃, 陈露. 玉米野生种基因组研究进展及应用[J]. 生物技术通报, 2024, 40(3): 14-24. |
| [11] | 李灿, 蒋湘宁, 盖颖. 日本落叶松LkF3H2基因克隆及调控类黄酮代谢功能研究[J]. 生物技术通报, 2024, 40(2): 245-252. |
| [12] | 华炫, 田博雯, 周欣彤, 江梓涵, 王诗琦, 黄倩慧, 张健, 陈艳红. 旱柳SmERF B3-45的克隆及耐盐功能研究[J]. 生物技术通报, 2024, 40(12): 124-135. |
| [13] | 殷子薇, 红雨. 玫瑰红球菌NB1对玉米的耐盐促生效应及其全基因组研究[J]. 生物技术通报, 2024, 40(12): 193-207. |
| [14] | 王晶, 张晓磊, 白玉, 盛宇欣, 关海涛, 温洪涛. 不同玉米转化体通用PCR检测体系建立[J]. 生物技术通报, 2024, 40(12): 34-44. |
| [15] | 田锦, 张月秋, 张华, 陈子言, 田璐, 王颢潜, 高芳瑞, 梁晋刚, 陈红. 转基因玉米浙大瑞丰8特异性定性PCR检测方法研究[J]. 生物技术通报, 2024, 40(12): 45-52. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||