








生物技术通报 ›› 2024, Vol. 40 ›› Issue (7): 259-272.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0116
沈真辉1,2,3,4( ), 曹瑶1,2,3,4, 杨林雷1,2,3,4, 罗祥英1,2,3,4, 子灵山1,2, 陆青青1,2, 李荣春1,2,3,4(
), 曹瑶1,2,3,4, 杨林雷1,2,3,4, 罗祥英1,2,3,4, 子灵山1,2, 陆青青1,2, 李荣春1,2,3,4( )
)
收稿日期:2024-01-31
出版日期:2024-07-26
发布日期:2024-07-30
通讯作者:
李荣春,教授,硕士生导师,研究方向:食用菌栽培与资源评价;E-mail: rongchunli@126.com作者简介:沈真辉,硕士,中级农艺师,研究方向:食用菌基础分子生物学;E-mail: 1947843434@qq.com
基金资助:
SHEN Zhen-hui1,2,3,4( ), CAO Yao1,2,3,4, YANG Lin-lei1,2,3,4, LUO Xiang-ying1,2,3,4, ZI Ling-shan1,2, LU Qing-qing1,2, LI Rong-chun1,2,3,4(
), CAO Yao1,2,3,4, YANG Lin-lei1,2,3,4, LUO Xiang-ying1,2,3,4, ZI Ling-shan1,2, LU Qing-qing1,2, LI Rong-chun1,2,3,4( )
)
Received:2024-01-31
Published:2024-07-26
Online:2024-07-30
摘要:
【目的】 探究金耳和毛韧革菌麦角硫因生物合成途径。【方法】 利用PCR扩增技术分别克隆金耳和毛韧革菌的麦角硫因合成酶基因Egt1和Egt2并利用生物信息学软件分析其功能;高效液相色谱技术鉴定2个物种的组氨酸三甲基内盐中间产物、麦角硫因及其含量。【结果】 成功克隆得到了2个物种Egt1和Egt2基因的完整DNA序列。生物信息学分析表明,2个物种的Egt1均含有EgtD和SAM依赖性甲基转移酶等功能结合域,Egt2均含有磷酸吡哆醛(LPL)和半胱氨酸脱硫酶的结合位点; Egt1和Egt2与裂殖酵母和粗糙脉孢菌等模型真菌具有相似的功能域和底物结合位点,表明Egt1和Egt2可能与这些模式真菌具有相似的基因功能。高效液相色谱法分析表明,金耳芽孢(JEYB)、毛韧革菌发酵液(ShFJY)、菌丝体(ShJST)及金耳子实体(JEZST)中均含有组氨酸三甲基内盐和麦角硫因,并且金耳子实体麦角硫因含量最高(113.19 μg/g),分别是金耳芽孢、毛韧革菌发酵液和毛韧革菌菌丝体的7.45倍、26.14倍和27.74倍。【结论】 首次鉴定了金耳和毛韧革菌的Egt1和Egt2基因。推测金耳和毛韧革菌的生物合成途径都是由组氨酸在Egt1酶催化形成组氨酸三甲基内盐,再由Egt1酶催化形成海西烯半胱氨酸亚砜,最后由Egt2酶催化最终形成麦角硫因。
沈真辉, 曹瑶, 杨林雷, 罗祥英, 子灵山, 陆青青, 李荣春. 金耳和毛韧革菌麦角硫因生物合成基因的克隆及生物信息学分析[J]. 生物技术通报, 2024, 40(7): 259-272.
SHEN Zhen-hui, CAO Yao, YANG Lin-lei, LUO Xiang-ying, ZI Ling-shan, LU Qing-qing, LI Rong-chun. Cloning and Bioinformatics Analysis of the Ergothioneine Biosynthesis Genes in Naematelia aurantialba and Stereum hirsutum[J]. Biotechnology Bulletin, 2024, 40(7): 259-272.
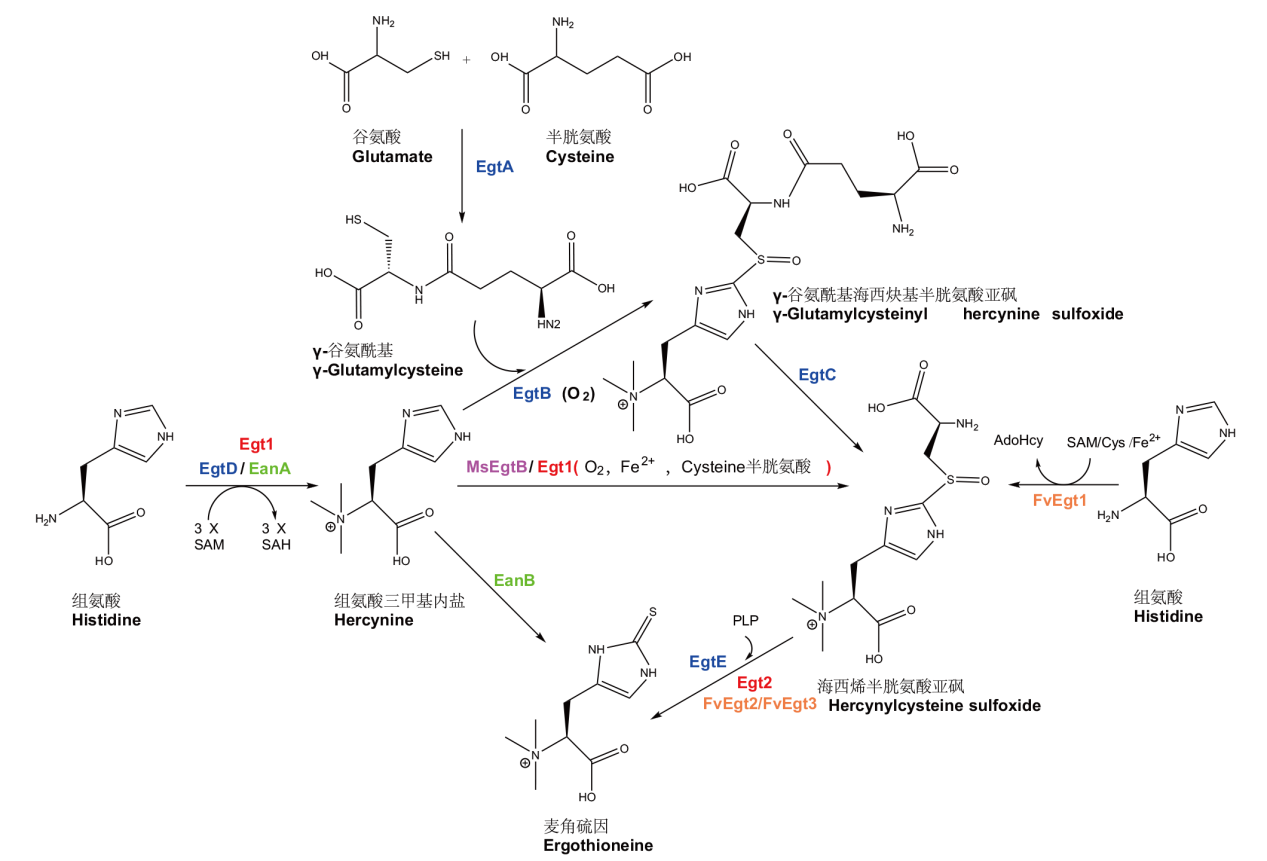
图1 微生物麦角硫因生物合成途径 SAM,SAH(或AdoHcy),Cys和PLP分别表示s-腺苷甲硫氨酸,S腺苷同型半胱氨酸,半胱氨酸和磷酸吡哆醛
Fig. 1 Biosynthetic pathways of ergothioneine in microorganism SAM, SAH (or AdoHcy), Cys and PLP refers to s-adenosylmethionine, S-adenosylhomocysteine, cysteine and pyridoxal phosphate respectively
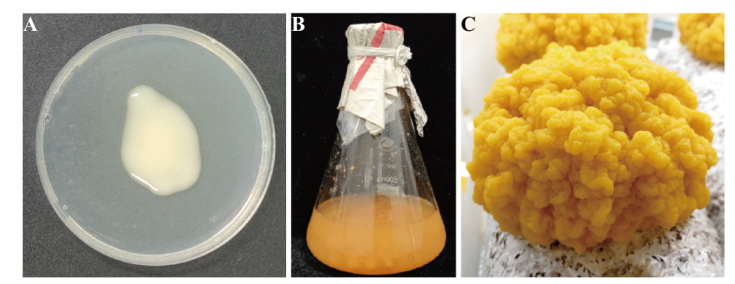
图2 不同样品形态特征 A:金耳芽孢(JEYB);B:毛韧革菌液体菌种(ShFJY+ShJST);C:金耳子实体(JEZST)
Fig. 2 Morphological characteristics of different samples A: N. aurantialba blastospore (JEYB);B: S. hirsutum liquid culture(ShFJY+ShJST);C: N. aurantialba fruiting body(JEZST)
| 序号Serial No. | 引物名称 Primer name | 序列 Sequence(5'-3') | 退火温度 Annealing temperature/℃ |
|---|---|---|---|
| 1 | ShEgt1-F | TGCGTCGGTCTCCTTTCTT | 53 |
| 2 | ShEgt1-R | CGGAGCAGAACAGATTTATCG | |
| 3 | ShEgt2-F | CACCACCACACATCACAGTC | 56 |
| 4 | ShEgt2-R | GACAAGTTCACAACGAGCAT | |
| 5 | NaEgt1-F | TGTCTGTCTCCTCCATCCA | 56 |
| 6 | NaEgt1-R | TAGTCCCAGGCTCATCACA | |
| 7 | NaEgt2-F | GTATCGCTGTTTACCACCTGT | 56 |
| 8 | NaEgt2-F | TCTACTCATCCTTCGTCGG |
表1 金耳和毛韧革菌麦角硫因生物合成基因引物信息
Table 1 Primer information for ergothioneine biosynthetic genes of N. aurantialba and S. hirsutum
| 序号Serial No. | 引物名称 Primer name | 序列 Sequence(5'-3') | 退火温度 Annealing temperature/℃ |
|---|---|---|---|
| 1 | ShEgt1-F | TGCGTCGGTCTCCTTTCTT | 53 |
| 2 | ShEgt1-R | CGGAGCAGAACAGATTTATCG | |
| 3 | ShEgt2-F | CACCACCACACATCACAGTC | 56 |
| 4 | ShEgt2-R | GACAAGTTCACAACGAGCAT | |
| 5 | NaEgt1-F | TGTCTGTCTCCTCCATCCA | 56 |
| 6 | NaEgt1-R | TAGTCCCAGGCTCATCACA | |
| 7 | NaEgt2-F | GTATCGCTGTTTACCACCTGT | 56 |
| 8 | NaEgt2-F | TCTACTCATCCTTCGTCGG |
| 序号Serial No. | 分析软件Analysis software | 用途Application | 网址Web site |
|---|---|---|---|
| 1 | ORF | CDS序列预测CDS sequence prediction | |
| 2 | Gene Structure Display Server | 绘制基因结构图Drawing a genetic structure map | |
| 3 | NCBI-Blast | 基因同源性分析Gene homology analysis | |
| 4 | ExPASy- ProtParam | 分析蛋白的理化性质Analyzing the physicochemical properties of proteins | |
| 5 | SignalP4.1 | 信号肽预测Prediction of signal peptide | |
| 6 | PSORT II | 亚细胞定位Subcellular localization | |
| 7 | CCD | 保守结构域预测Conservative structural domain prediction | |
| 8 | PSIPRED | 二级结构预测Secondary structure of protein prediction | |
| 9 | SWISS-MODEL | 三级结构预测Tertiary structure prediction | |
| 10 | Phyre2 | 三级结构配体预测Tertiary structure ligand prediction | |
| 11 | MEGA7.0 | 系统进化树分析Phylogenetic analysis | |
表2 本研究所用的生物信息学软件
Table 2 Bioinformatics software used in this study
| 序号Serial No. | 分析软件Analysis software | 用途Application | 网址Web site |
|---|---|---|---|
| 1 | ORF | CDS序列预测CDS sequence prediction | |
| 2 | Gene Structure Display Server | 绘制基因结构图Drawing a genetic structure map | |
| 3 | NCBI-Blast | 基因同源性分析Gene homology analysis | |
| 4 | ExPASy- ProtParam | 分析蛋白的理化性质Analyzing the physicochemical properties of proteins | |
| 5 | SignalP4.1 | 信号肽预测Prediction of signal peptide | |
| 6 | PSORT II | 亚细胞定位Subcellular localization | |
| 7 | CCD | 保守结构域预测Conservative structural domain prediction | |
| 8 | PSIPRED | 二级结构预测Secondary structure of protein prediction | |
| 9 | SWISS-MODEL | 三级结构预测Tertiary structure prediction | |
| 10 | Phyre2 | 三级结构配体预测Tertiary structure ligand prediction | |
| 11 | MEGA7.0 | 系统进化树分析Phylogenetic analysis | |
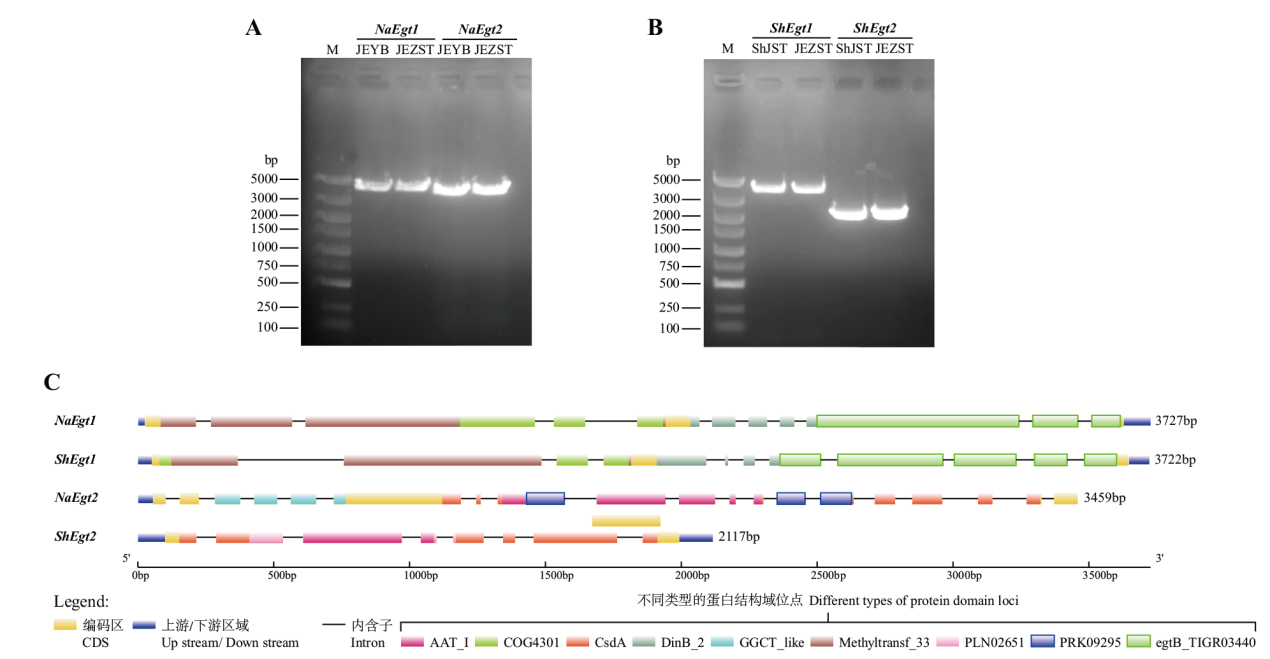
图3 金耳和毛韧革菌的Egt1和Egt2基因PCR扩增验证(A,B)及基因结构分析(C) M:DL2000 marker;ShJST:毛韧革菌菌丝体;JEYB:金耳芽孢;JEZST:金耳子实体
Fig. 3 Verification of PCR amplification of Egt1 and Egt2 genes of N. aurantialba and S. hirsutum(A, B)and gene structure analysis(C) M: DL2000 marker; ShJST: S. hirsutum mycelium; JEYB: N. aurantialba blastospore ; JEZST: N. aurantialba fruiting body
| 基因 Gene | CDS长度CDS length/bp | 蛋白质长度 Protein length | 蛋白质分子量Molecular weight/kD | 等电点 Isoelectric point | 总平均亲水系数Grand average of hydropathicity (GRAVY) | 信号肽 Signal peptide | 细胞中分布 Location |
|---|---|---|---|---|---|---|---|
| NaEgt1 | 2952 | 984 | 110.32 | 5.30 | -0.479 | 无No | 细胞质Cytoplasmic |
| ShEgt1 | 2565 | 885 | 99.03 | 5.19 | -0.528 | 无No | 细胞质Cytoplasmic |
| NaEgt2 | 2178 | 726 | 81.61 | 5.87 | -0.368 | 无No | 细胞质Cytoplasmic |
| ShEgt2 | 1383 | 461 | 51.46 | 5.68 | -0.238 | 无No | 细胞质Cytoplasmic |
表3 两个物种Egt1和Egt2理化性质分析
Table 3 Analysis of physical and chemical properties of Egt1 and Egt2 in two species
| 基因 Gene | CDS长度CDS length/bp | 蛋白质长度 Protein length | 蛋白质分子量Molecular weight/kD | 等电点 Isoelectric point | 总平均亲水系数Grand average of hydropathicity (GRAVY) | 信号肽 Signal peptide | 细胞中分布 Location |
|---|---|---|---|---|---|---|---|
| NaEgt1 | 2952 | 984 | 110.32 | 5.30 | -0.479 | 无No | 细胞质Cytoplasmic |
| ShEgt1 | 2565 | 885 | 99.03 | 5.19 | -0.528 | 无No | 细胞质Cytoplasmic |
| NaEgt2 | 2178 | 726 | 81.61 | 5.87 | -0.368 | 无No | 细胞质Cytoplasmic |
| ShEgt2 | 1383 | 461 | 51.46 | 5.68 | -0.238 | 无No | 细胞质Cytoplasmic |

图4 五个真菌Egt1和Egt2蛋白三级结构及结合位点分析 A:5个真菌Egt1和Egt2(Egt3)的三级结构比较;B:Egt1与咪唑(红色箭头)和醋酸根离子(蓝色箭头)结合;C:Egt1与s-腺苷甲硫氨酸(SAM)的结合;D:EgtD与Fe3+的结合;E:Egt1与半胱氨酸(红色箭头)结合;F:Egt2与S-巯基半胱氨酸(红色箭头)结合;G-H:Egt2与两个磷酸吡哆醛(PLP)位点结合。SpEgt1、NcEgt1、FvEgt1、SpEgt2、NcEgt2、FvEgt2及FvEgt3分别表示裂殖酵母、粗糙脉孢霉、金针菇的麦角硫因生物合成关键酶(登录号:CAA22334.2、XP_956324.3、QBB19872.1、NP_595091.1、A7UX13、QBB19873.1及QBB19874.1)
Fig. 4 Tertiary structure and binding site analysis of five fungal Egt1 and Egt2 proteins A: Comparison of the tertiary structures of five fungal Egt1 and Egt2(Egt3); B: Egt1 binds to imidazole(red arrow)and acetate ions(blue arrow); C: binding of Egt1 to s-adenosylmethionine(SAM); D: the combination of EgtD and Fe3+; E: Egt1 binds to cysteine(red arrow); F: Egt2 binds to S-mercaptocysteine; G-H: Egt2 binds to two pyridoxal phosphate(PLP)sites. SpEgt1, NcEgt1, FvEgt1, SpEgt2, NcEgt2, FvEgt2 and FvEgt3 are key enzyme for ergothioneine biosynthesis of S. pombe, N. crassa and F. filiformis, respectively(Accession number: CAA22334.2, XP_956324.3, QBB19872.1, NP_595091.1, A7UX13, QBB19873.1 and QBB19874.1).
| 蛋白 Protein | 物种 Species | 模板 Template | 覆盖度 Coverage/% | 可信度 Confidence/% | 功能域一致性 Functional domain identity/% | 结合位点 Binding site |
|---|---|---|---|---|---|---|
| Egt1 | 耻垢分枝杆菌 M smegmatis | c4uy5A | 51/46/55/36/73 | 100/100/100/100/100 | 34/32/26/24/35 | 咪唑和醋酸根离子 Imidazole and acetate ion |
| 大利什曼原虫 Leishmania major | d1xtpa | 23/35/38/-/59 | 96/98/99/-/98 | 31/30/18/-/19 | s-腺苷甲硫氨酸 S-adenosyl-methionine(SAM) | |
| 耐热分枝杆菌 Mycobacterium thermoresistibile | c4x8bA | 44/49/55/57/- | 100/100/100/100/- | 27/26/23/24/- | Fe3+离子 Fe3+ ion | |
| 嗜热氢单胞菌 Hydrogenimonas thermophila | c8khqD | 44/48/55/57/- | 100/100/100/100/- | 26/26/24/24/- | 半胱氨酸 Cysteine(Cys) | |
| Egt2 | 粗糙脉孢霉 N. crassa | c5utsC | 59/91/97/94/93/92 | 100/100/100/100/100/100 | 39/35/31/97/21/36 | - |
| 集胞藻属 Synechocystis sp. | d1elua | 56/88/95/90/90/89 | 99.9/100/100/100/100/100 | 20/19/16/16/18/16 | 磷酸吡哆醛和S-巯基半胱氨酸 Pyridoxal phosphate and S-mercaptocysteine |
表4 5种真菌的Egt1和Egt2蛋白三级结构预测信息
Table 4 Predicted information on the tertiary structure of Egt1 and Egt2 proteins from five fungal species
| 蛋白 Protein | 物种 Species | 模板 Template | 覆盖度 Coverage/% | 可信度 Confidence/% | 功能域一致性 Functional domain identity/% | 结合位点 Binding site |
|---|---|---|---|---|---|---|
| Egt1 | 耻垢分枝杆菌 M smegmatis | c4uy5A | 51/46/55/36/73 | 100/100/100/100/100 | 34/32/26/24/35 | 咪唑和醋酸根离子 Imidazole and acetate ion |
| 大利什曼原虫 Leishmania major | d1xtpa | 23/35/38/-/59 | 96/98/99/-/98 | 31/30/18/-/19 | s-腺苷甲硫氨酸 S-adenosyl-methionine(SAM) | |
| 耐热分枝杆菌 Mycobacterium thermoresistibile | c4x8bA | 44/49/55/57/- | 100/100/100/100/- | 27/26/23/24/- | Fe3+离子 Fe3+ ion | |
| 嗜热氢单胞菌 Hydrogenimonas thermophila | c8khqD | 44/48/55/57/- | 100/100/100/100/- | 26/26/24/24/- | 半胱氨酸 Cysteine(Cys) | |
| Egt2 | 粗糙脉孢霉 N. crassa | c5utsC | 59/91/97/94/93/92 | 100/100/100/100/100/100 | 39/35/31/97/21/36 | - |
| 集胞藻属 Synechocystis sp. | d1elua | 56/88/95/90/90/89 | 99.9/100/100/100/100/100 | 20/19/16/16/18/16 | 磷酸吡哆醛和S-巯基半胱氨酸 Pyridoxal phosphate and S-mercaptocysteine |

图5 不同物种Egt1(A)和Egt2(B)系统进化树分析 紫色标注表示毛韧革菌和金耳的Egt2序列比对结果;红色标注表示毛韧革菌与其他真菌的Egt2序列比对结果;蓝色标注表示金耳与其他真菌的Egt2序列比对结果
Fig. 5 Phylogenetic tree analysis of Egt1(A)and Egt2(B)in different species Purple colours indicate the results of Egt2 sequence comparison between S. hirsutum and N. aurantialba; red colours indicate the results of Egt2 sequence comparison between S. hirsutum and other fungi; blue colours indicate the results of Egt2 sequence comparison between N. aurantialba and other fungi
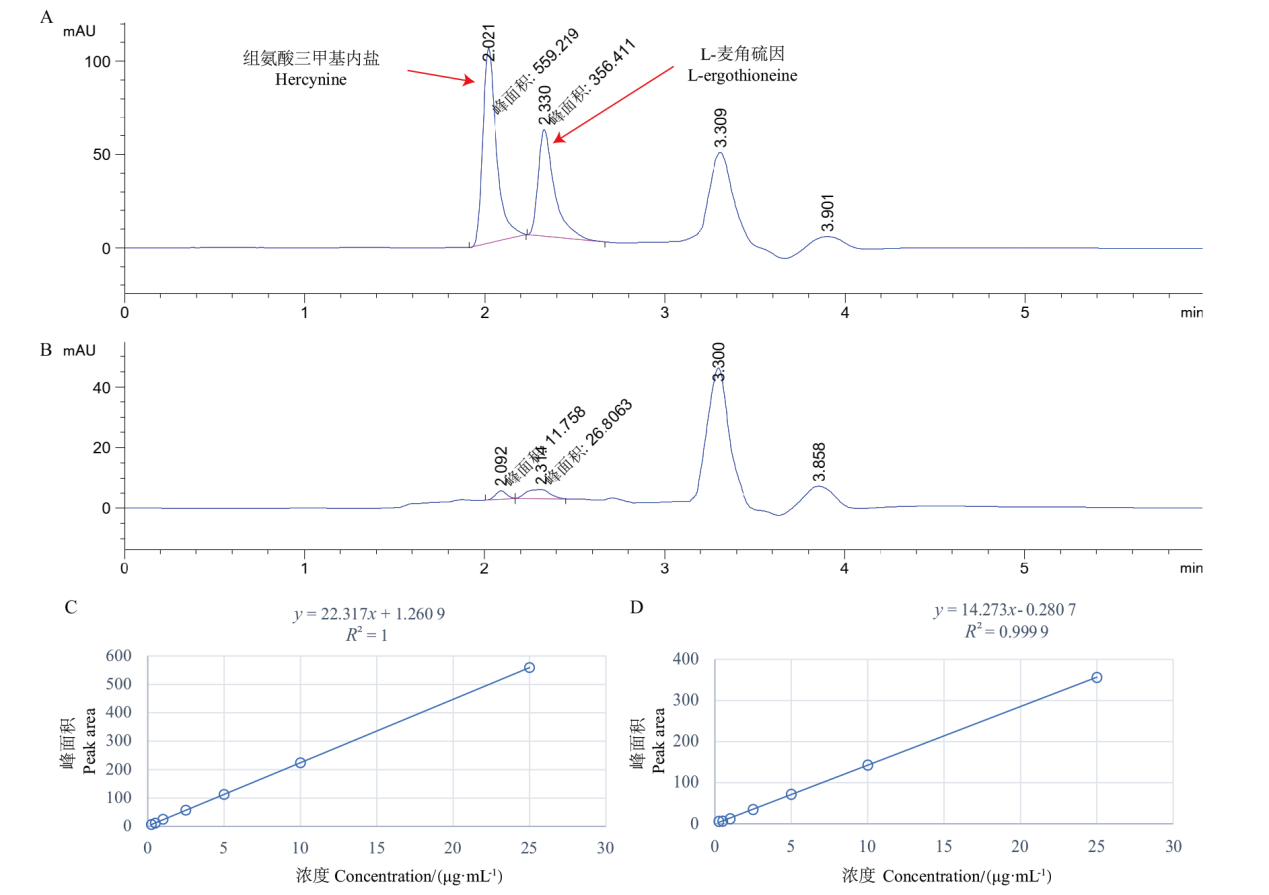
图6 标准品(A)和金耳子实体(JEZST)样品(B)的HPLC图谱,组氨酸三甲基内盐(C)和麦角硫因(D)标准曲线
Fig 6 Chromatograms of reference substances(A)and N. aurantialba fruiting body(JEZST)(B), standard curve of hercynine and L-ergothioneine
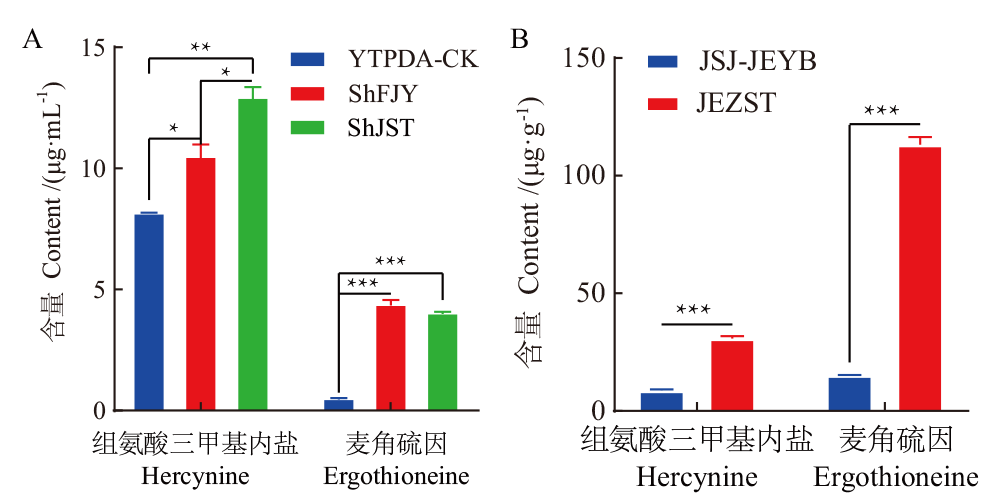
图7 不同样品组氨酸三甲基内盐和麦角硫因含量测定 A:液体样品;B:固体样品。YTPDA-CK:PDA液体培养基(对照);ShFJY:毛韧革菌发酵液;ShJST:毛韧革菌菌丝体;JSJ-JEYB:金耳芽孢;JEZST:金耳子实体。*,**和***分别表示在P<0.05、P<0.01和P<0.0001水平上差异显著
Fig. 7 Determination of hercynine and ergothioneine content in different samples A: Liquid sample; B: solid sample. YTPDA-CK: PDA liquid medium(control); ShFJY: S. hirsutum fermentation broth; ShJST: S. hirsutum mycelium; JSJ-JEYB: N. aurantialba blastospa; JEZST: N. aurantialba fruiting body. *, ** and *** indicate a significant difference at P<0.05, P<0.01 and P<0.0001, respectively
| [1] | Tanret C. Surune base nouvelle retiree du seigle ergote, L-ergoth-ioneine compt[J]. Rend Acad Sci, 1909, 149: 222-224. |
| [2] |
Franzoni F, Colognato R, Galetta F, et al. An in vitro study on the free radical scavenging capacity of ergothioneine: comparison with reduced glutathione, uric acid and trolox[J]. Biomed Pharmacother, 2006, 60(8): 453-457.
pmid: 16930933 |
| [3] | 林陈水, 付水星, 黎小军, 等. 一种稀有的天然氨基酸-麦角硫因[J]. 氨基酸和生物资源, 2006, 28(1): 63-67. |
| Lin CS, Fu SX, Li XJ, et al. Ergothioneine—a rare natural amino acid[J]. Amino Acids Biotic Resour, 2006, 28(1): 63-67. | |
| [4] | Speisky H, Gómez M, Carrasco-Pozo C, et al. Cu(I)-glutathione complex: a potential source of superoxide radicals generation[J]. Bioorg Med Chem, 2008, 16(13): 6568-6574. |
| [5] | 朱本占, 毛莉, 范瑞梅, 等. 天然抗氧化剂麦角硫因保护铜所致DNA和蛋白质氧化损伤的作用机理[J]. 科学通报, 2011, 56(27): 2283-2288. |
| Zhu BZ, Mao L, Fan RM, et al. Mechanism of protection by natural antioxidant ergothioneine against copper-induced oxidative damage to DNA and protein[J]. Chin Sci Bull, 2011, 56(27): 2283-2288. | |
| [6] | Markova NG, Karaman-Jurukovska N, Dong KK, et al. Skin cells and tissue are capable of using L-ergothioneine as an integral component of their antioxidant defense system[J]. Free Radic Biol Med, 2009, 46(8): 1168-1176. |
| [7] | 张萌萌, 张倩, 张国琛. 金针菇提取液中麦角硫因氨基酸的功能及其在食品工业中的应用前景[J]. 安徽农业科学, 2014, 42(11): 3385-3387, 3390. |
| Zhang MM, Zhang Q, Zhang GC. The function of ergothioneine in the extracts from Flammulina velutipes and its application prospects on food industry[J]. J Anhui Agric Sci, 2014, 42(11): 3385-3387, 3390. | |
| [8] | 张翠, 赵艳敏, 白淑芳, 等. HPLC法测定不同品种蘑菇中麦角硫因的含量[J]. 食品工业科技, 2013, 34(23): 307-310. |
| Zhang C, Zhao YM, Bai SF, et al. HPLC determination of ergothioneine in mushrooms of different species[J]. Sci Technol Food Ind, 2013, 34(23): 307-310. | |
| [9] | 木开代斯·买合木提, 陈建, 焦春伟, 等. L-麦角硫因生物合成与应用研究进展[J]. 天然产物研究与开发, 2022, 34(4): 713-721. |
| Mukaidaisi·MHMT, Chen J, Jiao CW, et al. Progress in biosynthesis and application of L-ergothioneine[J]. Nat Prod Res Dev, 2022, 34(4): 713-721. | |
| [10] | 刘琦, 毛雨丰, 廖小平, 等. 麦角硫因生物合成研究的新进展[J]. 生物工程学报, 2022, 38(4): 1408-1420. |
| Liu Q, Mao YF, Liao XP, et al. Recent progress in ergothioneine biosynthesis: a review[J]. Chin J Biotechnol, 2022, 38(4): 1408-1420. | |
| [11] | Seebeck FP. In vitro reconstitution of mycobacterial ergothioneine biosynthesis[J]. J Am Chem Soc, 2010, 132(19): 6632-6633. |
| [12] | Kamide T, Takusagawa S, Tanaka N, et al. High Production of Ergothioneine in Escherichia coli using the Sulfoxide Synthase from Methylobacterium strains[J]. J Agric Food Chem, 2020, 68(23): 6390-6394. |
| [13] | Burn R, Misson L, Meury M, et al. Anaerobic origin of ergothioneine[J]. Angew Chem Int Ed Engl, 2017, 56(41): 12508-12511. |
| [14] | Bello MH, Barrera-Perez V, Morin D, et al. The Neurospora crassa mutant NcΔEgt-1 identifies an ergothioneine biosynthetic gene and demonstrates that ergothioneine enhances conidial survival and protects against peroxide toxicity during conidial germination[J]. Fungal Genet Biol, 2012, 49(2): 160-172. |
| [15] | Pluskal T, Ueno M, Yanagida M. Genetic and metabolomic dissection of the ergothioneine and selenoneine biosynthetic pathway in the fission yeast, S. pombe, and construction of an overproduction system[J]. PLoS One, 2014, 9(5): e97774. |
| [16] |
Fujitani Y, Alamgir KM, Tani A. Ergothioneine production using Methylobacterium species, yeast, and fungi[J]. J Biosci Bioeng, 2018, 126(6): 715-722.
doi: S1389-1723(18)30295-0 pmid: 29910189 |
| [17] | Hu W, Song H, Sae Her A, et al. Bioinformatic and biochemical characterizations of C-S bond formation and cleavage enzymes in the fungus Neurospora crassa ergothioneine biosynthetic pathway[J]. Org Lett, 2014, 16(20): 5382-5385. |
| [18] | Cumming BM, Chinta KC, Reddy VP, et al. Role of ergothioneine in microbial physiology and pathogenesis[J]. Antioxid Redox Signal, 2018, 28(6): 431-444. |
| [19] | Yang XQ, Lin SX, Lin JD, et al. The biosynthetic pathway of ergothioneine in culinary-medicinal winter mushroom, Flammulina velutipes(agaricomycetes)[J]. Int J Med Mushrooms, 2020, 22(2): 171-181. |
| [20] | 梅保良, 刘琦, 姜文侠, 等. 营养因子强化麦角硫因生物合成的研究[J]. 食品研究与开发, 2015, 36(15): 108-112. |
| Mei BL, Liu Q, Jiang WX, et al. Study on the biosynthesis of L-ergothioneine by enhancement of nutritional factors[J]. Food Res Dev, 2015, 36(15): 108-112. | |
| [21] | Tepwong P, Giri A, Sasaki F, et al. Mycobial enhancement of ergothioneine by submerged cultivation of edible mushroom mycelia and its application as an antioxidative compound[J]. Food Chem, 2012, 131(1): 247-258. |
| [22] | Dubost NJ, Beelman RB, Royse DJ. Influence of selected cultural factors and postharvest storage on ergothioneine content of common button mushroom Agaricus bisporus(J. lge)imbach(agaricomycetideae)[J]. Int J Med Mushr, 2007, 9(2): 163-176. |
| [23] | Liang CH, Huang LY, Ho KJ, et al. Submerged cultivation of mycelium with high ergothioneine content from the culinary-medicinal king oyster mushroom Pleurotus eryngii(higher basidiomycetes)and its composition[J]. Int J Med Mushrooms, 2013, 15(2): 153-164. |
| [24] |
Kalaras MD, Richie JP, Calcagnotto A, et al. Mushrooms: a rich source of the antioxidants ergothioneine and glutathione[J]. Food Chem, 2017, 233: 429-433.
doi: S0308-8146(17)30691-X pmid: 28530594 |
| [25] | Lin SY, Chien SC, Wang SY, et al. Submerged cultivation of My-celium with high ergothioneine content from the culinary-medicinal golden oyster mushroom, Pleurotus citrinopileatus(higher basidiomycetes)[J]. Int J Med Mushrooms, 2015, 17(8): 749-761. |
| [26] | 杨林雷, 李荣春, 曹瑶, 等. 金耳的学名及分类地位考证[J]. 食药用菌, 2020, 28(4): 252-255, 276. |
| Yang LL, Li RC, Cao Y, et al. Research on the scientific Name and taxonomic status of “Jin Er”[J]. Edible Med Mushrooms, 2020, 28(4): 252-255, 276. | |
| [27] |
Liu XZ, Wang QM, Göker M, et al. Towards an integrated phylogenetic classification of the Tremellomycetes[J]. Stud Mycol, 2015, 81: 85-147.
doi: 10.1016/j.simyco.2015.12.001 pmid: 26955199 |
| [28] | 郭正堂. 中国韧革菌(Ⅲ)[J]. 植物研究, 1987, 7(3): 85-112. |
| Guo ZT. Stereaceae in China(Ⅲ)[J]. Bull Bot Res, 1987, 7(3): 85-112. | |
| [29] | 罗晓莉, 张沙沙, 曹晶晶, 等. 云南3种胶质食用菌营养成分分析与蛋白质营养价值评价[J]. 食品工业科技, 2021, 42(14): 328-333. |
| Luo XL, Zhang SS, Cao JJ, et al. Analysis of nutritional components and evaluation of protein nutritional value of three kinds of gelatinous edible fungi in Yunnan[J]. Sci Technol Food Ind, 2021, 42(14): 328-333. | |
| [30] | 曹瑶, 李荣春, 杨林雷, 等. 工厂化栽培金耳的氨基酸组成及蛋白质营养评价[J]. 食药用菌, 2021, 29(2): 152-156. |
| Cao Y, Li RC, Yang LL, et al. Amino acid composition and nutritional evaluation of protein of industrial cultivated Naematelia aurantialba[J]. Edible Med Mushrooms, 2021, 29(2): 152-156. | |
| [31] | 曹瑶, 李荣春, 杨林雷, 等. 工厂化栽培金耳的营养成分测定及品质评价[J]. 食药用菌, 2021, 29(4): 318-322. |
| Cao Y, Li RC, Yang LL, et al. Basic nutrition component analysis and quality evaluation of industrial cultivated “Jiner”(Naematelia aurantialba)[J]. Edible Med Mushrooms, 2021, 29(4): 318-322. | |
| [32] | 曹瑶, 杨晓君, 杨林雷, 等. 金耳浆对挂面品质的影响[J]. 食品工程, 2023(3): 27-30, 34. |
| Cao Y, Yang XJ, Yang LL, et al. Effects of the fresh mushroom of N. aurantialba on the noodle quality[J]. Food Eng, 2023(3): 27-30, 34. | |
| [33] | 沈真辉, 罗祥英, 赵仙伟, 等. 利用转录组分析光照对金耳子实体转色的影响[J]. 食用菌学报, 2023, 30(6): 12-27. |
| Shen ZH, Luo XY, Zhao XW, et al. Transcriptome analysis revealed impact of light quality on coloration of Naematelia aurantialba fruiting bodies[J]. Acta Edulis Fungi, 2023, 30(6): 12-27. | |
| [34] | 杨林雷, 李荣春, 曹瑶, 等. 金耳及金耳多糖的药用保健功效及其机理研究进展[J]. 食药用菌, 2021, 29(3): 176-182. |
| Yang LL, Li RC, Cao Y, et al. Research progress on the pharmacology and health care effects of Naematelia aurantialba and its polysaccharides[J]. Edible Med Mushrooms, 2021, 29(3): 176-182. | |
| [35] | 何容, 罗晓莉, 李建英, 等. 金耳研究现状与展望[J]. 食药用菌, 2019, 27(1): 41-47. |
| He R, Luo XL, Li JY, et al. Research status and prospect of Tremella aurantialba[J]. Edible Med Mushrooms, 2019, 27(1): 41-47. | |
| [36] | 孙涛, 姜浩, 王燕玲, 等. 金耳多糖的研究进展[J]. 中国食品学报, 2022, 22(8): 386-397. |
| Sun T, Jiang H, Wang YL, et al. Research advances on Naematelia aurantialba polysaccharides[J]. J Chin Inst Food Sci Technol, 2022, 22(8): 386-397. | |
| [37] | Yan YH, Wang MT, Chen N, et al. Isolation, structures, bioactivities, application and future prospective for polysaccharides from Tremella aurantialba: a review[J]. Front Immunol, 2022, 13: 1091210. |
| [38] |
Halliwell B, Cheah IK, Tang RMY. Ergothioneine - a diet-derived antioxidant with therapeutic potential[J]. FEBS Lett, 2018, 592(20): 3357-3366.
doi: 10.1002/1873-3468.13123 pmid: 29851075 |
| [39] | 薛天凯, 赵艳敏, 林纪伟, 等. 正交设计优化平菇下脚料中麦角硫因的提取工艺[J]. 食品研究与开发, 2017, 38(3): 40-45. |
| Xue TK, Zhao YM, Lin JW, et al. Optimization of the extraction technique of ergothioneine from mushroom scraps by orthogonal method[J]. Food Res Dev, 2017, 38(3): 40-45. | |
| [40] | 赵艳敏, 雷智东, 刘成航, 等. 高效液相色谱法测定多种真菌中麦角硫因含量[J]. 食品研究与开发, 2016, 37(2): 117-119. |
| Zhao YM, Lei ZD, Liu CH, et al. Determination of ergothioneine in multiple species of fungi by HPLC[J]. Food Res Dev, 2016, 37(2): 117-119. | |
| [41] | 陈柏雄. 蛹虫草虫草素和麦角硫因生物合成基因功能及其调控机制研究[D]. 广州: 华南农业大学, 2020. |
| Chen BX. The biosynthetic genes fuction and regulatory mechanism of cordycepin and ergothioneine in Cordyceps militaris[D]. Guangzhou: South China Agricultural University, 2020. | |
| [42] | 林金德. 侧耳属食用菌麦角硫因合成酶基因的挖掘及其功能研究[D]. 广州: 华南农业大学, 2020. |
| Lin JD. Mining and function study of ergothioneine synthase genes of Pleurotus edible fungus[D]. Guangzhou: South China Agricultural University, 2020. | |
| [43] | 余颖豪. 灰树花麦角硫因生物合成基因的功能研究[D]. 广州: 华南农业大学, 2020. |
| Yu YH. Elucidation of the function of ergothioneine biosynthesis genes from Grifola frondosa[D]. Guangzhou: South China Agricultural University, 2020. | |
| [44] | Jeong JH, Cha HJ, Ha SC, et al. Structural insights into the histidine trimethylation activity of EgtD from Mycobacterium smegmatis[J]. Biochem Biophys Res Commun, 2014, 452(4): 1098-1103. |
| [1] | 庞梦真, 徐汉琴, 刘海燕, 宋娟, 王佳涵, 孙丽娜, 姬佩梅, 尹泽芝, 胡又川, 赵晓萌, 梁闪闪, 张泗举, 栾维江. 水稻黄化早抽穗突变体 hz1 的基因鉴定及功能分析[J]. 生物技术通报, 2024, 40(7): 125-136. |
| [2] | 黄丹, 姜山, 彭涛. 褐角苔FfCYP98基因克隆及其功能分析[J]. 生物技术通报, 2024, 40(7): 273-284. |
| [3] | 何玙冰, 付振浩, 李仁瀚, 刘秀霞, 刘春立, 杨艳坤, 李业, 白仲虎. 利用代谢工程在酿酒酵母中高效合成2-萘乙醇[J]. 生物技术通报, 2024, 40(7): 99-107. |
| [4] | 李梦然, 叶伟, 李赛妮, 张维阳, 李建军, 章卫民. Lithocarols类化合物生物合成基因litI的表达及其启动子功能分析[J]. 生物技术通报, 2024, 40(6): 310-318. |
| [5] | 张美玉, 赵玉斌, 王灵云, 宋元达, 赵新河, 任晓洁. 微藻破囊壶菌产功能性脂肪酸DHA研究进展[J]. 生物技术通报, 2024, 40(6): 81-94. |
| [6] | 胡锦锦, 李素贞, 马旭辉, 柳小庆, 谢珊珊, 江海洋, 陈茹梅. 玉米花青素生物合成代谢调控[J]. 生物技术通报, 2024, 40(6): 34-44. |
| [7] | 王玉书, 赵琳琳, 赵爽, 胡琦, 白慧霞, 王欢, 曹业萍, 范震宇. 大白菜BrCYP83B1基因的克隆及表达分析[J]. 生物技术通报, 2024, 40(6): 152-160. |
| [8] | 郝思怡, 张君珂, 王斌, 曲朋燕, 李瑞得, 程春振. 香蕉ELF3的克隆与表达分析[J]. 生物技术通报, 2024, 40(5): 131-140. |
| [9] | 杜泽光, 任少文, 张凤勤, 李梅兰, 李改珍, 齐仙惠. 大白菜BrMLP328的克隆、表达及功能验证[J]. 生物技术通报, 2024, 40(4): 122-129. |
| [10] | 刘换换, 杨立春, 李火根. 北美鹅掌楸LtMYB305基因的克隆及功能分析[J]. 生物技术通报, 2024, 40(4): 179-188. |
| [11] | 钟匀, 林春, 刘正杰, 董陈文华, 毛自朝, 李兴玉. 芦笋皂苷合成相关糖基转移酶基因克隆及原核表达分析[J]. 生物技术通报, 2024, 40(4): 255-263. |
| [12] | 杨艳, 胡洋, 刘霓如, 殷璐, 杨锐, 王鹏飞, 穆霄鹏, 张帅, 程春振, 张建成. ‘红满堂’苹果MbbZIP43基因的克隆与功能研究[J]. 生物技术通报, 2024, 40(2): 146-159. |
| [13] | 任延靖, 张鲁刚, 赵孟良, 李江, 邵登魁. 白菜种子cDNA酵母文库的构建及BrTTG1互作蛋白的筛选及分析[J]. 生物技术通报, 2024, 40(2): 223-232. |
| [14] | 王俊芳, 黄秋斌, 张飘丹, 张彭湃. Surfactin的结构、生物合成及其在生物防治中的作用[J]. 生物技术通报, 2024, 40(1): 100-112. |
| [15] | 朱毅, 柳唐镜, 宫国义, 张洁, 王晋芳, 张海英. 西瓜ClPP2C3克隆及表达分析[J]. 生物技术通报, 2024, 40(1): 243-249. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||